Digital Therapeutics Vendor Risk Management: FDA Regulation and Clinical Evidence
Post Summary
Digital therapeutics (DTx) are transforming healthcare by offering software-based treatments for managing and preventing diseases. These FDA-regulated tools require rigorous clinical validation and vendor risk management to ensure safety, compliance, and effectiveness. Key challenges include navigating evolving regulations, verifying clinical evidence, and addressing cybersecurity risks.
Key Takeaways:
- FDA Oversight: DTx products are regulated under Software as a Medical Device (SaMD), with varying requirements for Class I, II, and III devices.
- Clinical Evidence: High-quality studies, often randomized with control groups, are critical for assessing a DTx product’s effectiveness.
- Risk Management: Healthcare organizations must evaluate vendor compliance, cybersecurity protocols, and software update processes.
Tools like Censinet RiskOps help streamline vendor assessments, ensuring compliance with FDA standards and addressing risks. As the DTx market grows, robust risk management practices are essential for maintaining patient safety and trust.
FDA Regulations for Digital Therapeutics
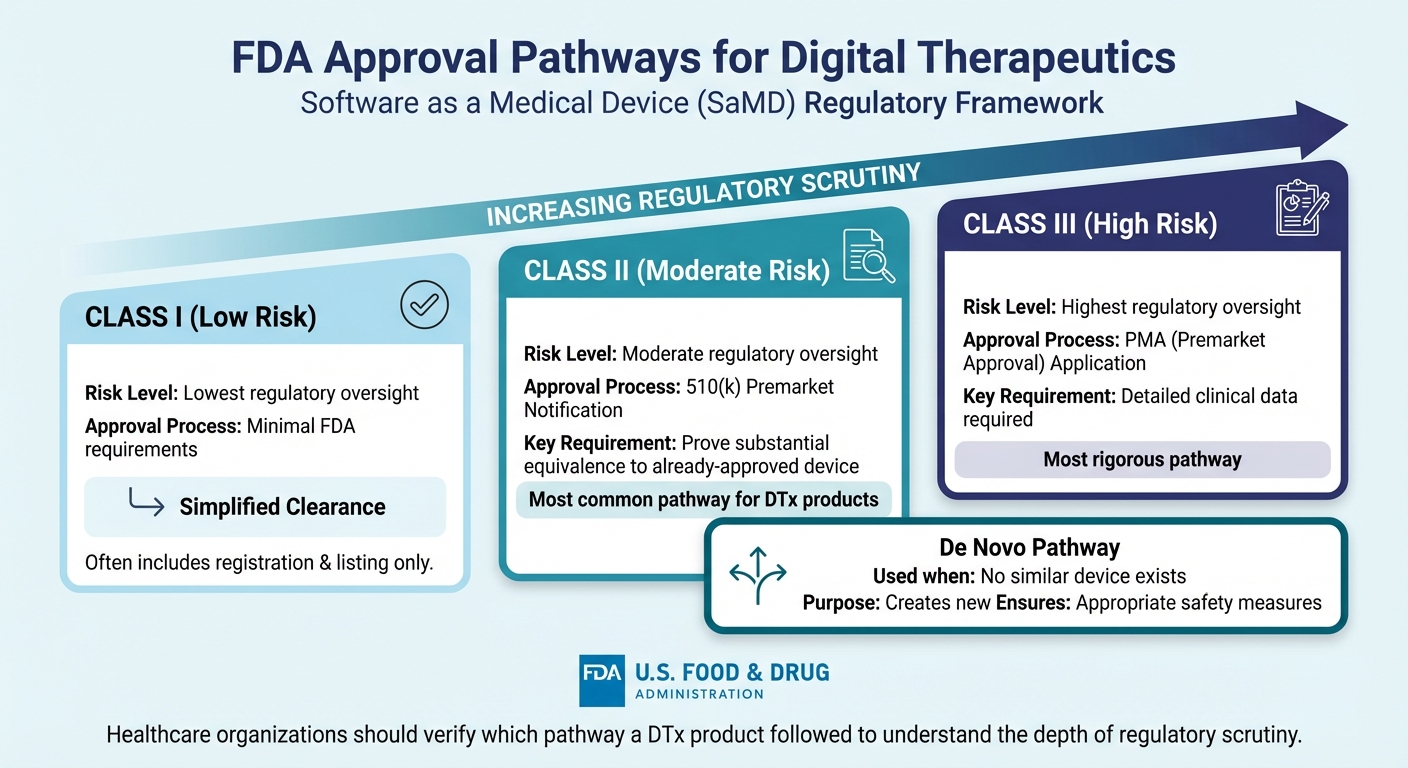
FDA Approval Pathways for Digital Therapeutics by Risk Classification
FDA Approval Pathways for DTx
The FDA oversees digital therapeutics under the category of Software as a Medical Device (SaMD) if the software is designed for medical purposes like diagnosing, treating, mitigating, or preventing disease - without being tied to a physical medical device [2][3][4][5].
The regulatory pathway for approval depends on the risk classification assigned to the digital therapeutic. Class I devices face the least regulatory oversight. Class II devices typically require a 510(k) premarket notification, which proves they are substantially equivalent to an already-approved device. Class III devices, considered the highest risk, must go through a PMA application supported by detailed clinical data. If no similar device exists, the De Novo pathway creates a new classification while ensuring appropriate safety measures.
Prescription Digital Therapeutics (PDTs) undergo a review process that mirrors prescription drugs but with different evidentiary standards due to their classification as medical devices rather than pharmaceuticals [6][7][8]. For healthcare organizations evaluating vendors, it’s essential to confirm the regulatory pathway a DTx product followed. This reveals the depth of scrutiny the product has undergone.
FDA Guidance Documents for Digital Health
The FDA has issued several guidance documents to clarify how digital therapeutics should be developed and assessed [9][10][11][12]. These guidelines cover key areas like SaMD classification, premarket submission requirements, clinical evidence expectations, cybersecurity measures, and the use of artificial intelligence in digital health tools.
Healthcare organizations can use these guidelines as a reference when evaluating vendors. Understanding FDA standards for cybersecurity, clinical validation, and software updates allows risk management teams to ask targeted questions and assess vendors effectively. This ensures vendors are not just claiming compliance but actively adhering to the FDA's specific recommendations. These documents serve as a foundation for verifying a vendor’s regulatory alignment.
Verifying FDA Compliance in Vendor Assessments
Don’t rely solely on marketing materials - review vendor documentation thoroughly. Healthcare organizations should request proof of FDA clearance, such as a 510(k) number, De Novo order, or PMA letter. The FDA provides publicly accessible databases where these submissions can be verified, along with decision summaries.
Ongoing compliance is just as critical as initial approval. Since digital therapeutics often involve software updates, significant changes may require additional regulatory submissions. Vendor evaluations should include inquiries about the vendor's quality management system, post-market monitoring processes, and how they determine whether updates trigger new FDA requirements. This ensures the product remains compliant with current regulations.
Clinical Evidence Requirements for Digital Therapeutics
Clinical Evidence Standards for DTx
When it comes to digital therapeutics (DTx), FDA clearance guarantees safety, but clinical evidence is what truly demonstrates patient benefits. To assess a DTx product, healthcare organizations need to examine the quality of the clinical studies behind it. The most reliable evidence comes from randomized interventional studies that include proper control groups - whether that's active, placebo, or no-intervention - to pinpoint the DTx's actual impact.
Blinding is another key element in ensuring unbiased outcome assessments. Studies should clearly define their primary outcome measures and include secondary endpoints to thoroughly evaluate the intervention's effects. Safety is equally critical in this process. Comprehensive reporting of adverse events and oversight by an independent Data Monitoring Committee (DMC) are essential to maintaining research integrity [13].
These rigorous study standards provide a solid framework for evaluating the clinical evidence behind DTx products.
Where to Verify Clinical Evidence
Healthcare organizations can verify clinical evidence by consulting trusted public registries like ClinicalTrials.gov, which offers detailed insights into study protocols, designs, and outcomes. Each study listed on the platform is assigned a unique NCT number, providing transparency about the study's objectives, intervention models, allocation methods, masking techniques, and outcome measures.
In addition to registries, FDA decision summaries for products cleared through pathways like 510(k), De Novo, or PMA offer valuable information. Cross-referencing these summaries with peer-reviewed publications helps ensure that the reported data is consistent. For additional insights, patient registries and observational studies can provide real-world evidence that complements clinical trial findings [13].
Evidence Gaps in Emerging DTx Products
While established DTx products often meet rigorous standards, emerging ones - especially in areas like digital mental health - can present evidence gaps. Some newer products enter the market based on preliminary studies without randomized controls or rely on nonrandomized allocation methods. These approaches can introduce bias, making it harder to draw reliable conclusions. A "Not Applicable" tag on ClinicalTrials.gov often indicates a departure from traditional FDA drug development phases, signaling the need for closer scrutiny of the study design.
Transparency is another hurdle. While the National Library of Medicine ensures that submissions to ClinicalTrials.gov are clear and consistent, it does not verify the scientific validity of the data. This responsibility falls on the study sponsor. Digging into participant flow data - like how many participants enrolled, completed, or dropped out - can uncover usability issues or challenges in real-world effectiveness. As these gaps come to light, they should prompt a reassessment of risks, especially as new data becomes available [13].
Risk Domains for Digital Therapeutics Vendors
When managing digital therapeutics (DTx) vendors, it's crucial to not only focus on regulatory and clinical validations but also to understand the risks associated with these technologies.
Regulatory and Compliance Risks
For a DTx product to fall under the FDA's oversight, it must meet the definition of a device as outlined in section 201(h) of the FD&C Act. This includes software designed for diagnosing, curing, mitigating, treating, or preventing diseases, as well as software that impacts body structure or function. However, certain low-risk software functions may be excluded from this classification [4][2].
Compliance with HIPAA is another key consideration. Since DTx vendors often handle protected health information (PHI), ensuring adherence to federal privacy laws is critical. This involves securing Business Associate Agreements (BAAs) and conducting regular audits to confirm compliance with HIPAA and state-specific privacy regulations. A clear and predictable regulatory framework is essential for protecting patients and maintaining trust in DTx solutions [1].
Addressing these regulatory and compliance requirements lays the groundwork for tackling other interconnected risks, such as cybersecurity.
Cybersecurity and Privacy Risks
Once compliance is established, the focus shifts to protecting data and maintaining network security. The reliance on internet connectivity and network integration increases exposure to cybersecurity threats [14]. Since cybersecurity directly impacts the safety and effectiveness of a device, these risks must be managed collaboratively by manufacturers and healthcare organizations [14][15].
The FDA’s updated guidance on cybersecurity, issued on June 27, 2023, provides a framework for identifying vulnerabilities. Healthcare organizations should use this guidance during vendor evaluations to assess potential weaknesses and ensure robust protections are in place.
Clinical and Patient Safety Risks
Without proper validation, digital therapeutics can pose risks such as adverse events or inappropriate treatment [2]. The FDA applies a risk-based framework to regulate software that could impact public health [2]. To minimize these risks, it’s essential to confirm that each product is designed for its intended patient population and clinical application. This step ensures the safety and effectiveness of the therapeutic solution.
sbb-itb-535baee
Building a DTx Vendor Risk Management Program
Creating a well-structured risk management program for digital therapeutics (DTx) vendors is essential to address FDA compliance, clinical safety, data integrity, and cybersecurity concerns. To manage the unique challenges posed by DTx products, healthcare organizations need strong governance, standardized evaluation processes, and continuous monitoring systems.
Governance, Policies, and Vendor Inventory
Start by establishing a governance framework that clearly defines roles for evaluating DTx vendors. This framework should include cross-functional teams from IT, clinical departments, compliance, and legal. Policies should specify criteria for evaluating DTx products, using the FDA's risk-based classification system - Class I (low risk), Class II (moderate risk), and Class III (high risk). This ensures the evaluation rigor matches the product's intended use and technical features [4][2].
Maintain a centralized inventory of all DTx vendors and products. This inventory should document each product's regulatory status, clinical indications, patient populations, and integration points within your systems. Such a resource simplifies vendor oversight and ensures nothing slips through the cracks. Early communication with the FDA is also important to clarify regulatory requirements and align with their expectations for digital health technologies. This detailed inventory sets the foundation for systematic risk assessments, as discussed in the next section.
Standardized Assessment Frameworks
Your assessment processes should align with FDA guidance and established frameworks like those from NIST. A key principle in FDA guidance is the "purpose-built" approach, which requires digital health technologies to be validated for their specific trial objectives and patient populations. Your framework should assess whether each DTx product meets these standards for its intended clinical application.
Standardized workflows are essential for evaluating regulatory compliance, cybersecurity, and clinical evidence. These workflows enable healthcare organizations to systematically address these critical aspects. Customizable assessment templates can also help target DTx-specific risk areas while maintaining consistency across vendor evaluations. This structured approach naturally leads to the need for ongoing oversight, which is covered in the next section.
Continuous Monitoring and Reassessment
DTx software often involves AI-driven updates, making continuous monitoring critical to ensure performance and safety [2][1]. Implement pre-defined change control plans for AI/ML-based DTx to manage future software updates and algorithm changes while maintaining patient safety [1].
The FDA's move toward a "Total Product Lifecycle" approach emphasizes ongoing monitoring of real-world performance and rapid product improvements [1]. Your monitoring program should track software updates, regulatory changes, and new clinical data. Regular security audits are vital to address risks introduced by software updates. Clinical evaluations should continue throughout the product's lifecycle, including post-market studies to reassess safety, learn from incidents, and adapt if a product's risk profile changes [16].
Conclusion
Digital therapeutics (DTx) are reshaping healthcare delivery, offering innovative ways to treat and manage various conditions. However, they also bring a unique set of challenges, particularly in vendor risk management. The FDA's risk-based regulatory frameworks play a key role in ensuring patient safety, but the rapid advancements in AI-driven DTx products continue to push the boundaries of traditional oversight.
As of December 2024, there are 192 FDA-approved DTx devices, with the market expected to grow to $12.1 billion by 2026 [17][2][10]. This growth underscores the need for a robust approach to vendor risk management, focusing on areas like regulatory compliance, clinical validation, cybersecurity, and patient safety.
"FDA has recognized for decades that software is not risk free. Software can result in adverse events, mistreatment, lack of treatment, or other errors across many disease areas."
To ensure patient outcomes remain a top priority, healthcare organizations must adopt specialized tools and systematic processes for managing DTx vendors. This includes evaluating clinical evidence, monitoring ongoing software updates, and addressing the unique risks posed by these technologies.
Censinet RiskOps offers a centralized solution for these challenges. It streamlines vendor assessments, ensures alignment with FDA requirements, and supports continuous monitoring. With its AI-driven capabilities, Censinet simplifies vendor questionnaire processes, summarizes clinical evidence, and routes critical findings to the right stakeholders, including AI governance committees. This approach addresses the complex regulatory, cybersecurity, and clinical risks associated with DTx.
In an era of rapid digital health innovation, effective vendor risk management isn't optional - it's essential. By implementing structured evaluation processes and leveraging purpose-built platforms, healthcare organizations can unlock the potential of digital therapeutics while safeguarding patient safety and meeting regulatory demands.
FAQs
What are the biggest challenges in managing vendor risks for digital therapeutics?
Managing vendor risks in digital therapeutics involves tackling a range of challenges. These include ensuring FDA compliance, confirming the clinical validity of therapeutic solutions, and addressing cybersecurity concerns related to data transmission. Organizations also need to adapt to the shifting classification of digital therapeutics, safeguard patient data privacy, and define clear roles and responsibilities, especially when working with decentralized teams.
Healthcare providers face additional obstacles, such as navigating insurance reimbursement processes and dealing with the regulatory complexities of the U.S. healthcare system. To ensure digital therapeutics are safe, effective, and meet compliance standards, proactive risk assessment and strong management strategies are essential.
How does the FDA regulate digital therapeutics classified as Software as a Medical Device (SaMD)?
The FDA oversees digital therapeutics through its Software as a Medical Device (SaMD) framework, organizing them into three risk-based categories: Class I (low risk), Class II (moderate risk), and Class III (high risk). The higher the risk classification, the more stringent the regulatory scrutiny to ensure both safety and effectiveness.
While most SaMDs are evaluated through established medical device pathways, certain low-risk software may fall under the FDA's "enforcement discretion." This means they aren't actively regulated, offering some flexibility. This balance helps ensure patient safety while encouraging advancements in digital health technologies.
How can healthcare organizations evaluate the clinical evidence of a digital therapeutic product?
When evaluating a digital therapeutic product, healthcare organizations need to ensure its safety, effectiveness, and reliability are backed by strong clinical evidence. This means the product should be validated within the appropriate patient populations, with data collected in a consistent and high-quality manner to support its claims.
It's crucial to focus on clinical outcomes that meet regulatory standards and demonstrate practical, real-world relevance. This approach confirms the product not only complies with regulations but also provides measurable and meaningful benefits to patients.
Related Blog Posts
- Building Vendor Risk Frameworks for Healthcare IT
- Medical Device Vendor Risk Management: FDA Compliance and Patient Safety Best Practices
- Medical Device Vendor Risk Management: FDA Compliance and Patient Safety Best Practices
- Clinical Decision Support System Vendor Risk: Bias, Accuracy, and Patient Safety


