DEA Compliance for Controlled Substance Vendors: Risk Management and Oversight
Post Summary
Handling controlled substances comes with strict DEA regulations to prevent misuse and illegal distribution. Vendors like manufacturers, distributors, and pharmacies must comply with the Controlled Substances Act (CSA) through proper registration, recordkeeping, and security measures. Non-compliance can lead to severe penalties, including fines or license suspension.
Here’s what you need to know:
- Registration: Every location handling controlled substances must have a DEA registration.
- Recordkeeping: Maintain accurate, detailed logs for all transactions, with separate records for Schedule I-II drugs.
- Storage: Use secure safes, alarms, and surveillance for controlled substances, especially higher schedules.
- Risk Management: Monitor suspicious orders, conduct regular audits, and report incidents promptly using DEA forms like Form 41.
Technology like AI-powered platforms can simplify compliance by automating inventory tracking and detecting diversion risks. Staying compliant protects public health and ensures your operations remain uninterrupted.
DEA Compliance Four Pillars Framework for Controlled Substance Vendors
Core DEA Compliance Requirements for Vendors
Navigating DEA compliance involves mastering three key areas: registration and licensing, recordkeeping and inventory management, and storage and security standards. Each area comes with specific rules that vendors must follow to legally handle controlled substances.
Registration, Renewal, and Licensing
To handle controlled substances, vendors must obtain and periodically renew a DEA registration [2][4]. Here's the catch: every principal physical location where controlled substances are managed needs its own DEA registration [2][4]. So, if a distributor has warehouses in multiple states, each facility must be registered separately.
Another layer to consider is state-level controlled substance registration (CSR) or licensing. This step is often required before applying for federal DEA registration [3][4]. Essentially, vendors need to meet all state requirements first, ensuring both state and federal authorities keep a close watch on controlled substance activities.
Once registration is in place, the focus shifts to maintaining accurate and detailed records.
Recordkeeping and Inventory Management
DEA compliance doesn’t stop at registration - it demands meticulous recordkeeping. Vendors must document every transaction involving controlled substances, and any gaps or errors in these records could lead to license suspension or fines [5]. Records need to be kept for at least two years after the substances are fully used, transferred, or disposed of. Copies inspected by the DEA must be retained for an additional two years [5].
For controlled substances, the DEA requires that records for Schedule I-II substances be stored separately from those for Schedule III-IV substances [5]. This segregation simplifies inspections and minimizes errors.
Jack Teitelman, a retired DEA Supervisory Special Agent and CEO of Titan Group, emphasizes the value of bound logbooks, stating that "the use of bound controlled substance logbooks such as the third edition of the AAHA Controlled Substance Logs meet the minimum requirements for use in all 50 US states and Canada" [7].
Bound logbooks are preferred over loose-leaf versions to prevent tampering, as loose pages can be easily removed [7]. Each log must include detailed information such as the DEA registrant, controlled substance name, stock container ID, storage location, amounts received, and usage details [5]. The physical inventory must always match the log records, and vendors are required to conduct an initial inventory when starting operations, followed by annual physical inventories to meet the DEA’s biennial requirements [5].
In October 2024, Wolters Kluwer introduced their Sentri7 Drug Diversion solution, which uses AI to identify drug diversion patterns by reconciling transactions. This tool highlights how technology can simplify controlled substance management while improving accuracy [6].
Storage and Security Standards
After ensuring proper documentation, vendors must implement robust physical security measures to protect controlled substances. The DEA’s requirements, detailed in 21 CFR Part 1301 (Sections 1301.72-1301.76), outline the necessary security controls and procedures [8][9].
For Schedule II substances, safes or vaults are typically required. Other measures include restricted access areas, alarm systems to detect unauthorized entry, and surveillance systems to monitor storage locations. These requirements vary depending on the substance schedule and the type of facility. Access to these areas must be limited to authorized personnel only.
Records should be stored alongside the controlled substances to ensure they’re readily accessible during inspections [5]. Keeping documents organized and easy to retrieve is vital for demonstrating compliance to DEA investigators during onsite reviews [6]. This approach ensures transparency and accountability in handling controlled substances.
Risk Management: Preventing Diversion and Responding to Incidents
Effective risk management goes beyond just meeting registration, recordkeeping, and storage requirements. It involves proactive strategies to prevent diversion, respond swiftly to incidents, and refine processes continually. These practices work hand in hand with compliance measures to strengthen overall security.
Suspicious Order Monitoring (SOM) Programs
A solid Suspicious Order Monitoring (SOM) program starts with a clear and detailed controlled substance policy and procedure manual. This manual should outline Standard Operating Procedures (SOPs) for every stage of the supply chain, including ordering, receiving, and inventorying controlled substances. To bolster security, vendors can implement practical safeguards such as:
- Conducting employee background checks, including routine re-screenings and drug testing.
- Installing physical security measures like alarm systems and video surveillance in storage areas.
- Engaging third-party auditors or DEA Compliance Groups for mock audits - ideally every two years.
- Maintaining accurate biennial inventories as required by Title 21, Code of Federal Regulations Section 1304.
These steps create a strong foundation for identifying and addressing potential risks. However, even the best monitoring systems need to be paired with a well-prepared framework for responding to incidents.
Incident Response and Reporting
When theft or loss occurs, vendors must act quickly and follow DEA reporting protocols. In cases of accidental loss - such as a spill or broken container - DEA Form 41 must be completed. This process includes:
- Securing the area to prevent further loss.
- Documenting key details, such as the substance involved, the quantity lost, and the cleanup steps taken.
- Having the individual responsible for the loss, along with any witnesses, sign and date the form.
- Ensuring the DEA registrant signs the form, retains a copy for their records, and updates the disposition record to reflect the loss.
Prompt and accurate reporting is critical to maintaining compliance and mitigating further risks.
Continuous Improvement in Diversion Prevention
Preventing diversion is an ongoing effort that requires regular evaluation and updates. Assigning a dedicated compliance officer can help ensure that procedures are consistently reviewed and improved. Key steps include:
- Providing comprehensive staff education and mandatory training on controlled substance handling, loss reporting, and recognizing signs of substance abuse.
- Regularly updating employee access lists and credentials to limit exposure to potential risks.
- Leveraging predictive analytics and AI surveillance tools to identify emerging threats before they escalate.
Technology Solutions for DEA Compliance and Oversight
Technology has become a game-changer in simplifying compliance processes while bolstering security measures. With modern tools, tasks like recordkeeping, inventory tracking, and order monitoring are now automated, reducing human error and ensuring facilities are prepared for audits.
Centralized Compliance Management
Centralized platforms bring all compliance-related tasks into a single, easily accessible system. Digital logbooks and real-time inventory management replace outdated manual methods, instantly reconciling transactions and highlighting discrepancies. For organizations managing multiple locations, corporate portals provide a bird's-eye view of operations, streamline user access management, and generate consolidated reports that make DEA inspections less daunting.
Jack Titelman, CEO of DEA Compliance Company and a former DEA agent, emphasizes the importance of preparation: "Every medical facility that handles controlled substances can face a DEA audit at any time. I've personally seen hundreds of failed audits and their consequences - ranging from fines in the thousands of dollars to the loss of an entire business. I've also witnessed dozens of successful audits - and those outcomes never come down to luck. They're always the result of thorough preparation and the right systems in place." [11]
This centralized system also sets the stage for advanced analytics to refine how suspicious orders are monitored.
Advanced Analytics for Suspicious Order Monitoring
Artificial intelligence (AI) and machine learning are revolutionizing the way drug diversion is detected. For example, Wolters Kluwer's Sentri7 platform evaluates over 60 risk factors from various sources like Electronic Health Records (EHRs) and Automated Dispensing Cabinets (ADCs) to spot diversion patterns. With 90% of reconciliation tasks automated, the platform provides early warnings of potential issues - an essential feature when you consider the estimated 37,000 drug diversion incidents that occur annually in U.S. healthcare facilities [12].
These systems also analyze order patterns to flag unusual activity. By examining factors such as the ratio of controlled to non-controlled substances, unexpected product combinations, or items outside a facility’s typical scope, they can identify suspicious behavior. For instance, when Analysis Group collaborated with a pharmaceutical distributor, they sifted through over two terabytes of historical transaction data to develop statistical models capable of instantly detecting irregular orders [13].
Cybersecurity and DEA Compliance Integration
Cybersecurity has become a cornerstone of DEA compliance, addressing vulnerabilities inherent in manual processes. Protecting data related to controlled substances isn’t optional - it’s a regulatory requirement. DEA Electronic Prescriptions for Controlled Substances (EPCS) regulations mandate robust security measures, and 36 states in the U.S. now require EPCS for controlled substance prescriptions [14]. These measures include:
- Two-factor authentication (2FA): Must use DEA-approved methods.
- Biometric systems: Ensure minimal false matches (≤0.001).
- Hardware tokens: Meet FIPS 140-2 Security Level 1 standards.
Maintaining comprehensive audit trails is another key requirement. Systems should automatically log all prescription activity, access changes, and interactions, retaining these records for at least two years to prepare for DEA inspections [14]. Integration with state Prescription Drug Monitoring Programs (PDMPs) adds another layer of protection by identifying potential drug-seeking behavior before prescriptions are issued.
The DEA has also raised concerns about cyber attacks targeting healthcare and pharmaceutical organizations. This highlights the need for robust cybersecurity systems to safeguard sensitive data and ensure compliance with regulatory standards [15].
sbb-itb-535baee
Governance and Auditing in DEA Compliance Programs
Strong governance and regular audits are the foundation of a reliable DEA compliance program. Without clear accountability and consistent reviews, even the most advanced measures can falter under scrutiny. By combining compliance strategies with technological safeguards, governance and audits ensure the program remains solid and dependable.
Roles and Responsibilities
For effective DEA compliance, roles must be clearly defined across departments. Compliance officers oversee the entire program, managing everything from registration renewals to monitoring suspicious orders. IT teams handle the security systems that protect sensitive controlled substance data. Meanwhile, operations staff focus on daily tasks like recordkeeping and inventory management, ensuring all documentation is accurate. The DEA’s proposed Suspicious Order Monitoring framework highlights the importance of this collaboration: when a suspicious order is flagged, teams need to report it to the DEA’s centralized database and either decline the order or complete due diligence within seven days [1]. This coordinated effort between compliance, operations, and IT ensures no critical responsibilities are overlooked.
Internal Auditing and Self-Inspections
Internal audits are your first line of defense against compliance issues. These self-inspections offer a deep dive into a facility’s vulnerabilities, identifying risks for drug diversion and areas where regulatory standards might not be met. For instance, a large regional hospital system partnered with TITAN Group for unannounced mock DEA audits, which exposed weaknesses in their monitoring program. The hospital’s Corporate Director of Compliance Audits shared:
"Jack and his team provided best practices that enhanced our diversion monitoring, policies, and internal investigation skills" [16].
Such audits not only reveal gaps but also provide actionable steps to strengthen compliance.
Preparing for DEA Inspections
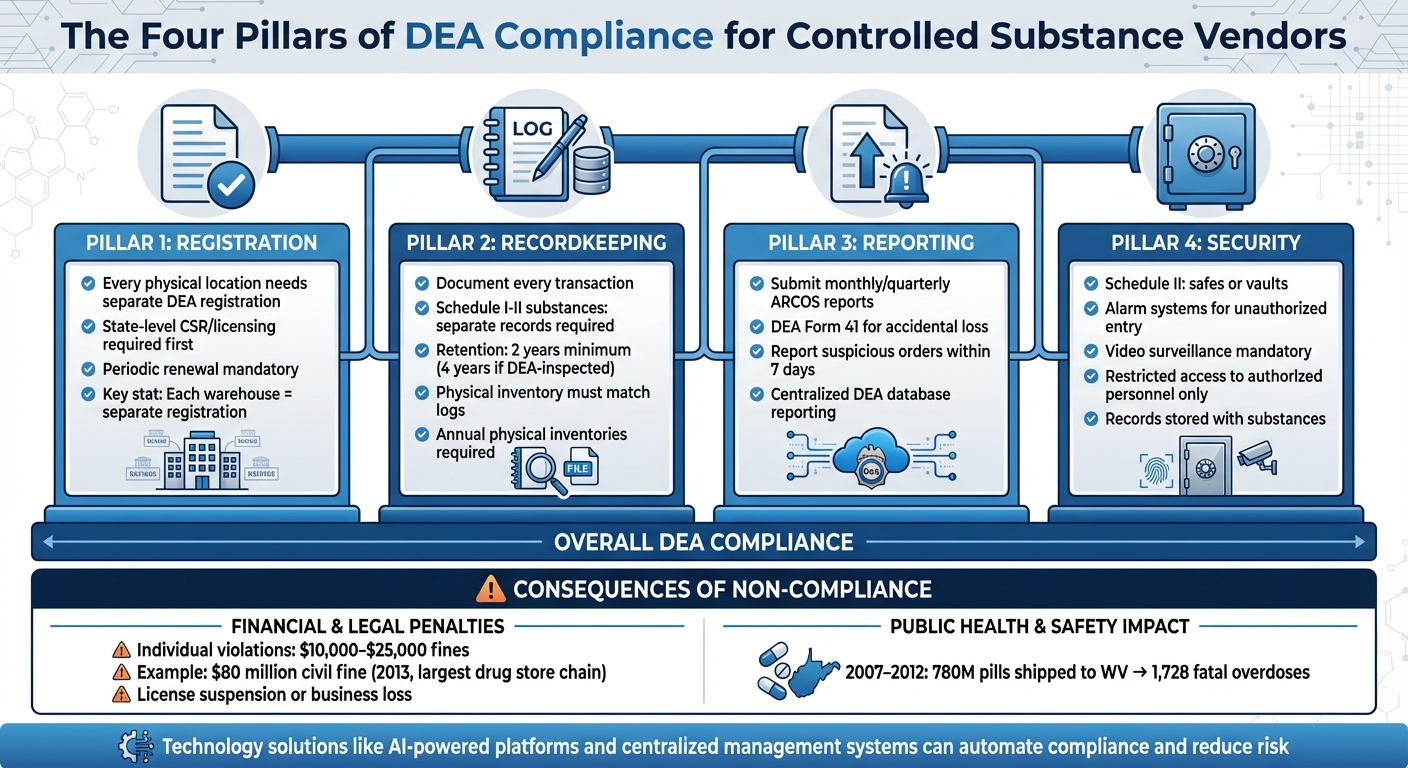
Being ready for a DEA inspection requires focusing on four key areas: registration, recordkeeping, reporting, and security [17]. Start by ensuring all DEA registrations are up to date for every location where controlled substances are stored. Keep detailed and accurate records for every step of the process, from receiving shipments to disposing of products. Submit timely reports - monthly or quarterly - through the Automation of Reports and Consolidated Orders System (ARCOS), which tracks controlled substance distribution and flags potential diversion risks [17].
Physical security measures are equally critical. Facilities need documented systems like cameras, alarms, locked gates, and restricted access. For Schedule III–V substances, storage must meet specific DEA standards, such as tamper-resistant hardware and self-closing doors [18]. Cathy Gallagher, Sr. Consultant at U.S. Compliance Solutions, IQVIA, offers this advice:
"Maintaining DEA compliance requires a lot of effort and paperwork, but if you make these four pillars the foundation for your controlled substance compliance program, you will have the tools in place to ensure compliance and survive your next inspection" [17].
Conclusion
Maintaining DEA compliance for controlled substance vendors is a continuous responsibility that requires constant attention, teamwork, and a proactive approach across your organization. As highlighted earlier, the foundation of an effective compliance program rests on four key areas: registration, recordkeeping, reporting, and security. These elements are essential, but compliance is not a fixed target - tactics used for diversion are always shifting [17][1].
The stakes are high. Between 2007 and 2012, 780 million hydrocodone and oxycodone pills were shipped to West Virginia, leading to 1,728 fatal overdoses in the state [1]. In 2013, the largest drug store chain in the U.S. faced $80 million in civil fines for failing to report suspicious orders [1]. Additionally, individual violations can result in fines ranging from $10,000 to $25,000, highlighting the serious financial and reputational risks tied to non-compliance [19]. These cases emphasize the critical need for comprehensive compliance strategies.
Technology plays a crucial role in strengthening DEA compliance efforts. For instance, the DEA's centralized database for suspicious order reporting - established under the Preventing Drug Diversion Act of 2018 - makes it easier to report suspicious activities and ensures investigators receive timely updates [1]. Advanced tools like the ARCOS distributor tool go a step further by identifying patterns that traditional methods might miss [1].
As the DEA has stated, "registrants are best situated to know their customers" [1]. This underscores the importance of evolving your compliance program through regular audits, updated protocols, robust security measures, accessible regulatory documents, and strict adherence to biennial inventory requirements [10]. Staying vigilant and adaptable is not just a best practice - it’s a necessity.
FAQs
What steps are involved in getting and renewing a DEA registration for controlled substances?
To get a DEA registration, you'll need to fill out the correct online form based on your role. For practitioners, use Form 224, and for researchers, it's Form 225. Make sure to provide all the required credentials, pay the applicable fee, and submit your application. After submission, you might need to undergo a DEA inspection before your registration is approved.
If you're renewing your registration, complete the online renewal process before it expires. Depending on your registration type, this could be either annually or every three years. Double-check that all your information is accurate, pay the renewal fee, and you'll stay compliant.
How can vendors use technology to ensure compliance with DEA regulations for controlled substances?
Technology is a game-changer for vendors working to meet DEA regulations for controlled substances. By introducing automated inventory management and digital recordkeeping, vendors can minimize errors and keep their records accurate and current. Tools like electronic ordering platforms make processes smoother and ensure adherence to DEA guidelines.
Real-time tracking adds another layer of oversight, allowing vendors to monitor controlled substances closely. Meanwhile, secure, tamper-proof documentation makes inspections and audits far more straightforward. These solutions not only streamline operations but also give vendors the tools they need to confidently comply with strict regulatory requirements.
What steps can controlled substance vendors take to prevent and address drug diversion?
To tackle drug diversion effectively, start by enforcing rigorous inventory controls, scheduling frequent audits, and carrying out detailed background checks on employees. Make sure all controlled substances are stored in secure locations, and use surveillance systems to keep an eye on who has access.
If you notice any suspicious activity, don’t hesitate - launch a detailed investigation and promptly report the issue to the proper authorities. Tools like tamper-evident packaging and automated tracking systems can play a key role in spotting and addressing diversion early, reducing risks and helping you stay compliant with DEA regulations.


