HITRUST Certification: Role of Automated Evidence Tools
Post Summary
Automated evidence tools simplify the HITRUST certification process by reducing manual effort, improving accuracy, and ensuring consistency in evidence collection. HITRUST certification, essential for U.S. healthcare organizations to protect patient data, requires extensive documentation to prove security controls are effective. Manual methods like spreadsheets and email threads are time-consuming and prone to errors, making automation a game-changer for efficiency and reliability.
Key Takeaways:
- HITRUST Certification Levels: e1 (44 controls, 1 year), i1 (182 controls, 1 year), and r2 (225+ controls, 2 years with midterm review).
- Challenges: Manual evidence collection is labor-intensive and risks delays or errors.
- Automated Tools: These integrate with systems like EHRs and vulnerability scanners, continuously pulling data, standardizing evidence, and organizing it for easier assessor review.
- Benefits: Faster assessments, better evidence quality, and improved compliance management tailored to healthcare needs.
Platforms like Censinet RiskOps™ centralize risk data, automate repetitive tasks, and streamline HITRUST certification, saving time and resources for healthcare organizations.
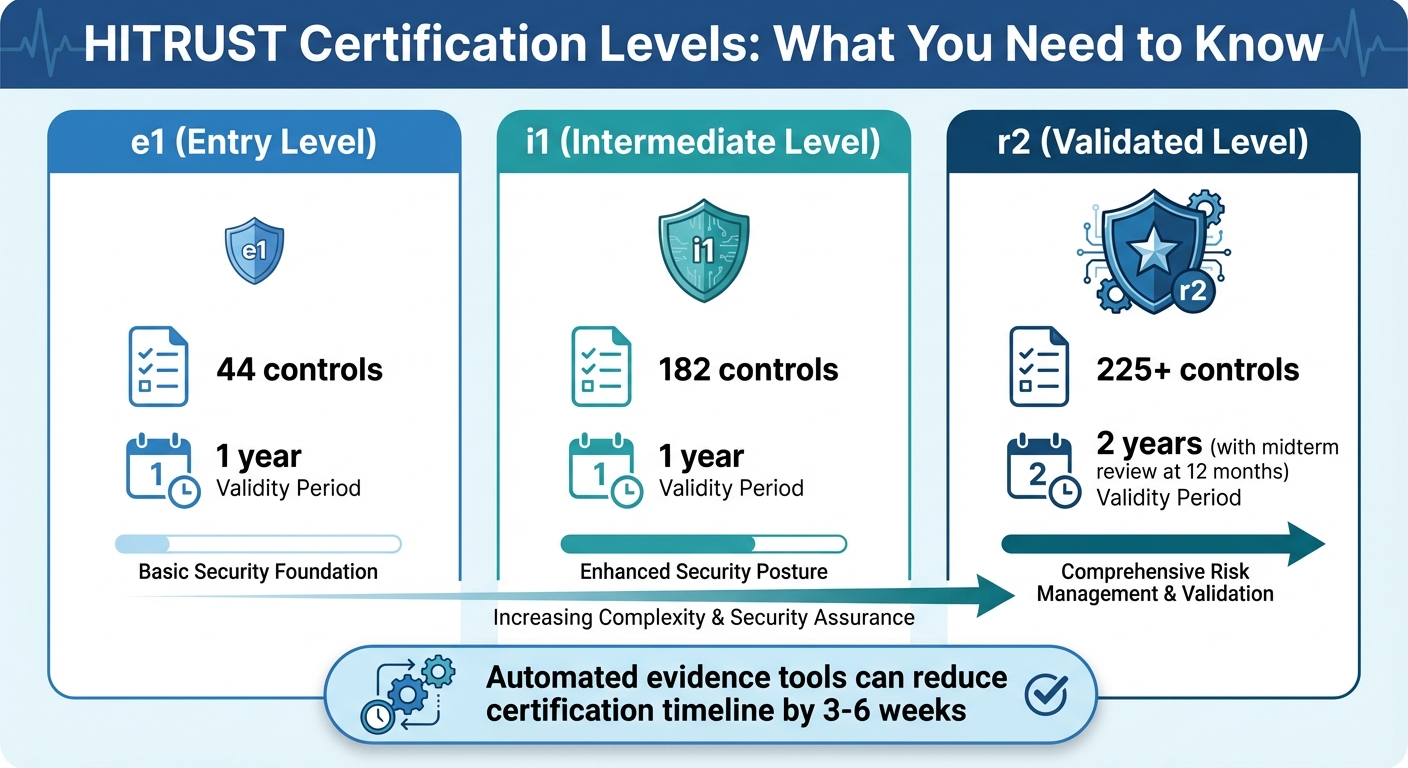
HITRUST Certification Levels: e1, i1, and r2 Requirements Comparison
How Automated Evidence Tools Support HITRUST Certification

What Are Automated Evidence Tools?
Automated evidence tools are software solutions that connect directly to an organization’s technical, security, and business systems to gather, standardize, and store the artifacts needed to prove control effectiveness [2][3]. Instead of relying on manual processes like exporting data, taking screenshots, or managing email threads, these tools act as a centralized hub that continuously pulls data from various systems [2].
These tools typically integrate with systems like identity and access management platforms, electronic health records (EHRs), endpoint protection software, vulnerability scanners, and ticketing systems. They can collect data on a schedule or when specific events occur, store evidence with version control, and provide role-based access for secure review by assessors and internal stakeholders. For healthcare organizations managing sensitive data like PHI or clinical applications, this means they can automatically capture evidence from sources such as EHR access logs, imaging systems, and population health tools. This automation reduces manual effort and creates a structured process that supports ongoing improvement during the HITRUST certification journey.
How Automation Improves Evidence Collection
Automation allows organizations to collect evidence continuously or nearly in real time by using APIs, log forwarders, and scheduled connectors that pull data at set intervals or in response to specific triggers [2][5]. For example, a healthcare organization could link its EHR access logs with its identity provider to ensure the tool constantly verifies that only authorized personnel access PHI [2][5].
This automated approach improves accuracy by pulling evidence directly from systems of record, minimizing risks like outdated screenshots or manual data entry errors [2][3]. It also ensures completeness by standardizing evidence requirements for each control and scheduling tasks to gather recurring artifacts, such as quarterly access reviews [2]. Additionally, it promotes consistency by using predefined templates and naming conventions [3][5].
HITRUST assessments often require evidence for a wide range of requirement statements across multiple domains. Manual evidence collection can lead to repeated requests for similar artifacts in different formats, creating inefficiencies [3][5]. Automated tools solve these issues by linking technical data sources to HITRUST controls, scheduling recurring collection tasks to prevent last-minute scrambles, and standardizing how evidence is labeled, tagged, and stored. This makes it easier for assessors to quickly locate the necessary evidence by control, asset, or domain [2][3][4].
Integration with HITRUST MyCSF
Many automated evidence tools are built to work alongside platforms like HITRUST MyCSF, which organizations use for assessments and control documentation. When integrated with HITRUST MyCSF, these tools address many challenges of manual evidence management. They map collected evidence to specific controls, simplifying the certification process. For instance, automated tools can pull data from vulnerability management systems, capturing scan results and remediation records, to provide an up-to-date view of control performance without relying on static screenshots [2][3]. This seamless integration with HITRUST MyCSF highlights how automated tools enhance efficiency and accuracy in achieving certification.
Benefits of Automated Evidence Tools for Healthcare Organizations
Faster Assessment and Certification
Automated evidence tools take the hassle out of managing control documentation, policies, and technical artifacts by centralizing everything in one place. No more chasing down spreadsheets, screenshots, or emails [2]. These tools automatically gather logs, configurations, and system settings from sources like EHRs, cloud platforms, and identity providers on a set schedule. This drastically cuts down on the manual effort needed for collecting data during HITRUST i1 or r2 assessments [2][3]. Tasks like scoping, gap analysis, and assessor reviews in MyCSF become much smoother and more efficient [2][3].
By consolidating tasks and eliminating duplicate requests, automation can trim 3–6 weeks off the readiness and remediation phases, significantly shortening the overall certification process [2]. For healthcare organizations undergoing regular annual or biennial HITRUST assessments, the ability to reuse validated evidence and control mappings makes subsequent certifications much quicker and easier [3]. A great example of this is Tower Health, which used Censinet RiskOps™ to free up three full-time employees (FTEs) who were reassigned to other roles, while the remaining two FTEs managed to conduct far more risk assessments than before [1]. Terry Grogan, CISO at Tower Health, highlighted the impact:
"Censinet RiskOps allowed 3 FTEs to go back to their real jobs! Now we do a lot more risk assessments with only 2 FTEs required." [1]
This streamlined and centralized approach aligns perfectly with the broader HITRUST certification process.
Better Evidence Quality and Consistency
Automation ensures evidence quality by pulling data directly from systems of record, reducing the chance of errors like outdated screenshots or manual entry mistakes. HITRUST and Rapid7 both stress that automated evidence collection and continuous validation can significantly reduce the "cost to assurance" by cutting down on manual efforts for control testing and evidence gathering [7]. Since HITRUST certification remains valid for two years - with an interim review required at the 12-month mark - maintaining up-to-date evidence is critical [2].
Automated tools make this easier by scheduling recurring tasks, like quarterly access reviews, and using standardized templates and naming conventions. This ensures that evidence is well-organized, labeled, and tagged consistently throughout the certification lifecycle. This level of organization simplifies both recertification and interim assessments. LevelBlue highlights how preparing evidence for a validated assessment involves a "detailed process that requires careful organization, proper documentation, and alignment with HITRUST's control framework," making automation a game-changer [6].
Meeting Healthcare-Specific Requirements
Healthcare organizations face unique challenges when it comes to protecting PHI and clinical data. As Matt Christensen, Sr. Director GRC at Intermountain Health, put it:
"Healthcare is the most complex industry... You can't just take a tool and apply it to healthcare if it wasn't built specifically for healthcare." [1]
Automated evidence tools tailored for the healthcare sector address these complexities by managing risks across patient data (PHI), medical devices, research, and the supply chain. They enable secure and efficient sharing of cybersecurity data within healthcare delivery organizations (HDOs) and their third-party vendors. This collaborative capability is essential for safeguarding PHI, medical devices, and clinical applications while ensuring smooth vendor risk management. These specialized features make automated tools an essential part of HITRUST compliance, especially for programs involving multiple business associates and third-party relationships.
sbb-itb-535baee
Using Censinet RiskOps™ for HITRUST Certification

Centralized Risk and Evidence Management
Censinet RiskOps™ acts as a single source of truth for managing risk and evidence data across your healthcare organization. It consolidates third-party vendor risk, enterprise risk assessments, and security documentation into one centralized system, eliminating the chaos of spreadsheets and email chains. When starting a HITRUST assessment, you can import the appropriate control set (e1, i1, or r2) and tailor it to your in-scope systems, vendors, and data flows. From there, RiskOps generates control-specific evidence tasks, assigns them to the appropriate team members, and monitors due dates through a centralized queue.
This streamlined setup ensures that resources like vendor security questionnaires, business associate agreements, medical device risk assessments, and PHI data-flow documentation - already collected for operational risk management - can be directly linked to HITRUST control requirements. RiskOps uniquely maps existing evidence to specific HITRUST controls, eliminating redundant efforts. When assessors begin their review, you can grant them controlled access to evidence, facilitate their questions, and track corrective action plans - all without the back-and-forth of endless email threads. This central repository also lays the groundwork for further automation in evidence management.
Automation and AI Features
Censinet RiskOps™ leverages automation and AI to streamline evidence collection while ensuring the human oversight required by HITRUST assessors. Tasks like distributing questionnaires, scoring vendor responses, tracking remediation deadlines, and sending renewal reminders are automated, helping you keep evidence up-to-date between certification cycles. The platform’s AI capabilities can complete security questionnaires almost instantly, summarize vendor evidence and documentation, and draft risk summary reports based on assessment data. This allows your team to focus on higher-level risk decisions instead of getting bogged down by administrative work.
However, all AI-suggested mappings and evidence require explicit human review and approval by control owners or compliance leads. The platform maintains detailed audit trails, recording approvals, review dates, and data sources. Automated checks can identify inconsistencies - such as conflicting system inventories or gaps between policy and evidence - so they can be resolved before the package is submitted to external assessors. This "human-in-the-loop" approach ensures that automation enhances decision-making without replacing critical oversight, delivering significant time savings while maintaining accuracy.
Supporting Healthcare Delivery Organizations
Censinet RiskOps™ is designed specifically for healthcare, addressing the unique challenges associated with PHI, clinical applications, and medical devices. The platform helps you map vendor security due diligence, medical device risk analyses, and PHI data-flow reviews directly to HITRUST requirements for third-party risk management, asset management, and information protection. It also enables you to track which vendors or internal systems impact high-risk PHI processes, critical clinical systems, or medical devices, allowing you to prioritize remediation efforts in alignment with HITRUST scoring criteria.
Operating within a collaborative network of healthcare organizations and over 50,000 vendors, the platform facilitates secure sharing of cybersecurity data across the healthcare community. This healthcare-specific focus is a game-changer for efficiency. Matt Christensen, Sr. Director GRC at Intermountain Health, highlights this point:
"Healthcare is the most complex industry... You can't just take a tool and apply it to healthcare if it wasn't built specifically for healthcare" [1].
Conclusion
Securing and maintaining HITRUST certification can significantly ease the operational and financial pressures faced by healthcare organizations. With automated evidence tools, the tedious process of manual evidence collection is replaced by streamlined solutions that gather, organize, and present documentation across numerous controls. Platforms like Censinet RiskOps™ centralize risk data, automate repetitive tasks, and maintain thorough audit trails, cutting down certification timelines by weeks.
The advantages go beyond just saving time. Automation enhances the quality and consistency of evidence, minimizing the chances of corrective action plans during validated assessments. This ensures that the evidence remains reliable and standardized throughout the certification process. Additionally, healthcare-specific platforms address risks unique to PHI, clinical applications, and medical devices, enabling organizations to focus remediation efforts on areas that directly influence HITRUST scoring. These combined efficiencies make it easier to embed compliance into daily operations.
FAQs
How do automated evidence tools help healthcare organizations achieve HITRUST certification?
Automated evidence tools make the HITRUST certification process much more manageable by integrating directly with healthcare systems to gather, organize, and verify compliance data. These tools connect to clinical, administrative, and security systems, allowing for real-time data extraction and ongoing monitoring.
By taking over the evidence collection process, these tools cut down on manual tasks, reduce the likelihood of errors, and ensure compliance documentation is both accurate and ready for audits. Censinet RiskOps™ takes this a step further, simplifying risk assessments and evidence management with a focus on the unique needs of healthcare organizations.
What are the advantages of using automated tools instead of manual methods for HITRUST certification?
Automated tools make the journey to HITRUST certification much smoother and faster. By cutting down on manual tasks, they help reduce errors and ensure evidence is collected consistently and accurately.
Another key benefit is continuous monitoring. These tools keep an eye on compliance requirements in real time, helping organizations spot and address gaps before they become bigger issues. With streamlined evidence management, companies can save time, work more efficiently, and approach the certification process with greater confidence.
How do automated evidence tools help healthcare organizations ensure data security and compliance?
Healthcare organizations face unique challenges when it comes to protecting sensitive data and staying compliant with regulations. Automated evidence tools are designed to address these challenges, using specialized technologies to ensure both security and compliance needs are met. These tools simplify the process of gathering and managing evidence, making it easier to handle sensitive information securely while adhering to standards like HITRUST certification.
With features like encryption, strict access controls, and continuous monitoring, these tools help safeguard patient data and other critical information. By automating these tasks, healthcare providers can minimize risks, save valuable time, and stay focused on delivering high-quality care - all without sacrificing security or compliance standards.


