Regulatory Trends in Healthcare Supply Chain Security 2025
Post Summary
Healthcare supply chains are under the microscope in 2025. With increasing vulnerabilities in global networks and reliance on regions like China and India for critical materials, disruptions can have far-reaching consequences. To address these risks, new regulations and laws are reshaping how healthcare organizations operate. Here’s what you need to know:
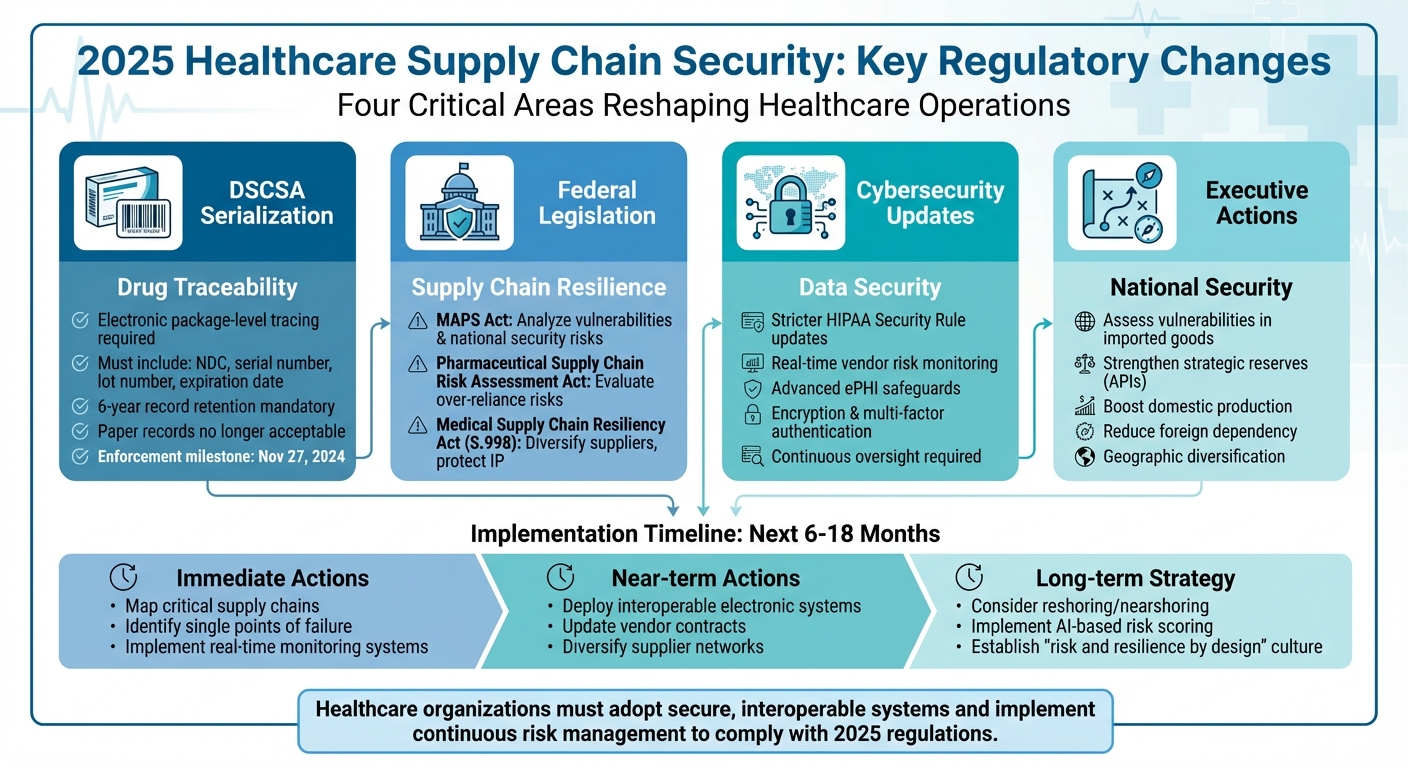
- Drug Supply Chain Security Act (DSCSA): Enforces electronic package-level tracing for prescription drugs, requiring serialization and digital record-keeping to combat counterfeit products.
- Federal Initiatives: Laws like the MAPS Act and Medical Supply Chain Resiliency Act aim to diversify suppliers, assess risks, and strengthen supply chain resilience.
- Cybersecurity Updates: Stricter HIPAA rules mandate advanced safeguards for electronic health information (ePHI) and real-time vendor risk monitoring.
- Executive Actions: Focus on reducing reliance on foreign sources and boosting domestic production of critical medical supplies.
Healthcare organizations must adopt secure, interoperable systems, diversify suppliers, and implement continuous risk management to comply with these changes and protect patient care.
Healthcare Supply Chain Security Compliance Roadmap 2025
Major Regulatory Changes Affecting Supply Chain Security in 2025
DSCSA Serialization and Traceability Requirements
The Drug Supply Chain Security Act (DSCSA) hit a key enforcement milestone on November 27, 2024, with the FDA allowing transition policies to extend implementation into 2025. Healthcare organizations are now required to implement electronic package-level tracing for prescription drugs throughout the supply chain [5].
Each prescription drug package must include a serialized product identifier containing the National Drug Code (NDC), a unique serial number, lot number, and expiration date. Trading partners must exchange transaction details electronically through secure systems - paper records are no longer acceptable. Additionally, all records must be preserved for six years [5].
Serialization data plays a critical role in identifying and isolating suspect or illegitimate products. Healthcare organizations must investigate flagged items, while wholesalers are required to verify returned medications with manufacturers before reselling them. Non-compliance could result in product quarantines, loss of trading partner authorization, enforcement actions, and even drug shortages that may directly affect patient care [5]. These stringent measures set the stage for broader legislative efforts aimed at fortifying the supply chain.
Federal Laws to Strengthen Supply Chains
Congress is moving forward with legislative initiatives to address vulnerabilities in the pharmaceutical supply chain. One such measure, the Mapping America's Pharmaceutical Supply (MAPS) Act, tasks the FDA and the Department of Defense with analyzing the U.S. pharmaceutical supply chain to identify weaknesses and potential national security risks using data-driven tools [3].
Another initiative, the Pharmaceutical Supply Chain Risk Assessment Act, calls for a comprehensive evaluation of risks stemming from over-reliance on single countries and lapses in quality control. The American Hospital Association has backed these efforts, pointing to past disruptions like the IV fluid shortage caused by Hurricane Helene as evidence of hidden vulnerabilities that only surface during crises [3].
The Medical Supply Chain Resiliency Act (S.998, 119th Congress) takes a trade-focused approach. It aims to improve the reliability of medical supplies by diversifying supplier networks, protecting intellectual property, and streamlining regulatory processes for cross-border movement during emergencies. These legislative efforts are complemented by executive actions that further prioritize supply chain resilience [4].
Executive Orders and National Security Priorities
Recent executive orders have elevated supply chain resilience to a national security priority. Federal agencies are now directed to assess vulnerabilities in imported goods and strengthen strategic reserves of critical materials, such as active pharmaceutical ingredients (APIs) [7]. These actions focus on bolstering domestic production, reducing reliance on high-risk foreign sources, and maintaining robust stockpiles of essential medical supplies.
For healthcare organizations, this means reevaluating supply chain strategies. Single-source or heavily concentrated foreign dependencies - particularly for APIs, sterile injectables, and critical devices - are under scrutiny. Organizations are encouraged to diversify geographically, build redundancy into their supply networks, and include emergency supply commitments in their contracts. These steps align with broader regulatory and executive initiatives aimed at securing the healthcare supply chain against future disruptions.
How Regulations Are Changing Healthcare Operations
New Standards for Third-Party Risk Management
Healthcare organizations are rethinking how they manage vendor relationships in response to stricter DSCSA and federal mandates. The days of relying on annual vendor reviews or static spreadsheets are over. Now, organizations must implement systems that provide real-time monitoring of vendor risks, including cybersecurity measures, product traceability, and compliance status.
Procurement processes are also evolving. Contract terms now include detailed regulatory requirements, ensuring vendors meet baseline security standards like encryption, multi-factor authentication, incident response protocols, and recovery objectives. Vendors are expected to undergo ongoing risk assessments that align with HIPAA and HITRUST standards rather than relying on outdated, one-time questionnaires.
The American Hospital Association has emphasized the importance of comprehensive supply chain mapping, noting that "vulnerabilities often only become apparent when the chain has been broken" [3]. This has led healthcare organizations to diversify their vendor portfolios, secure backup suppliers for critical products, and include emergency supply provisions in contracts. The proposed Medical Supply Chain Resiliency Act further supports these efforts by promoting diversified supplier networks and greater transparency in licensing and authorization processes [4]. These shifts are driving a stronger focus on secure data sharing and cyber resilience throughout the industry.
Increased Focus on Data Sharing and Cybersecurity
Regulations are also pushing healthcare organizations to adopt interoperable, cloud-based platforms for secure data exchange and real-time monitoring of electronic protected health information (ePHI). Just as DSCSA has highlighted the importance of traceability, these new mandates stress the need for constant vigilance in cybersecurity.
Proposed updates to the HIPAA Security Rule aim to enhance risk analysis, contingency planning, and security monitoring for ePHI, especially when third-party vendors are involved [6]. This means healthcare organizations must implement advanced safeguards and maintain continuous oversight of their supply chain systems to quickly detect and address any anomalies.
"Healthcare is the most complex industry... You can't just take a tool and apply it to healthcare if it wasn't built specifically for healthcare" [1].
sbb-itb-535baee
How Censinet RiskOps™ Addresses Supply Chain Security Requirements

Automated Third-Party and Enterprise Risk Assessments
Censinet RiskOps™ takes the hassle out of traditional risk assessment methods like questionnaires and spreadsheets by introducing an AI-driven, cloud-based risk exchange. This platform connects healthcare delivery organizations with a network of over 50,000 vendors and products, enabling real-time monitoring of supply chain risks. Terry Grogan, CISO at Tower Health, highlighted how this innovation has transformed their workflow:
"Censinet RiskOps allowed 3 FTEs to go back to their real jobs! Now we do a lot more risk assessments with only 2 FTEs required." [1]
By streamlining the process, the platform frees up valuable resources and allows teams to significantly increase the volume of risk assessments without additional staffing.
Using Censinet AI for Faster Risk Management
Censinet AI takes efficiency a step further by speeding up third-party risk assessments. Vendors can now complete security questionnaires in mere seconds, while the platform automatically compiles evidence and creates detailed reports. This blend of automation and human oversight not only slashes the time spent evaluating vulnerabilities but also supports a continuous, data-driven monitoring approach. Teams can set up configurable rules and workflows to maintain control over decisions related to supply chain security and compliance. Such agility is essential in meeting the industry's growing need for constant oversight, as required by evolving regulations. [1]
Coordinated Risk Management Between Organizations
Censinet RiskOps™ goes beyond automation by fostering collaboration. As the healthcare sector's first cloud-based risk exchange, it enables secure sharing of cybersecurity and risk data across a connected network of organizations. This collaborative approach enhances compliance efforts by improving supply chain visibility and supporting strategies that bolster resilience. James Case, VP & CISO at Baptist Health, shared how moving away from spreadsheets has transformed their risk management practices:
"Eliminating spreadsheets has enabled a broader, collaborative approach that enhances risk management." [1]
This networked model aligns with federal initiatives like the Medical Supply Chain Resiliency Act, which emphasizes diversification and strengthened oversight. By supporting these goals, Censinet RiskOps™ helps organizations adapt to regulatory demands while building a more resilient supply chain. [1]
Conclusion: Getting Ready for Future Supply Chain Security Challenges
What Healthcare Organizations Need to Know
The new DSCSA electronic traceability requirements, stricter HIPAA security measures, and federal initiatives like MAPS and the Medical Supply Chain Resiliency Act are reshaping the way healthcare organizations approach risk management. These changes demand constant vigilance and proactive measures to protect sensitive data and ensure compliance.
To keep up, healthcare organizations must prioritize ongoing third-party audits and real-time monitoring. The combination of global supply chain disruptions, tighter regulations, and rapid technological advancements makes this essential. Tools like Censinet RiskOps™ can help by providing automated risk assessments, AI-driven analytics, and access to a collaborative risk network that connects to over 50,000 vendors and products for real-time tracking and monitoring [1][2].
Next Steps: Creating Stronger Supply Chains
Healthcare leaders need to act quickly to adapt to these evolving regulations. Over the next 6–18 months, the focus should be on building supply chains that are both regulation-compliant and resilient. Start by mapping out critical supply chains and identifying potential vulnerabilities, such as single points of failure. Align these efforts with federal mapping and assessment strategies to ensure readiness.
Invest in interoperable electronic systems to manage DSCSA transaction data securely. Strong governance should be in place for serialization, verification, and recall workflows. Real-time tracking systems, such as digital tagging or blockchain, can streamline recall processes and improve transparency.
Additionally, predictive analytics can help anticipate disruptions caused by supplier delays, natural disasters, or geopolitical issues. Use these insights to create contingency plans, such as alternative sourcing or rerouting strategies. Contracts should also be updated to include clear expectations around DSCSA compliance, cyber incident cooperation, and regulatory audit support. Diversifying suppliers will further reduce reliance on any single source or region.
For long-term resilience, consider reshoring or nearshoring critical supplies. Implement digital product passports for seamless traceability and use AI-based risk scoring to continuously monitor vendor and supply chain vulnerabilities. By fostering a "risk and resilience by design" mindset and leveraging purpose-built platforms, healthcare organizations can navigate the changing regulatory landscape while staying prepared for future disruptions.
FAQs
What are the major updates to DSCSA serialization and traceability rules for 2025?
In 2025, the Drug Supply Chain Security Act (DSCSA) will implement tighter serialization standards to strengthen the pharmaceutical supply chain's security. Key updates include enhanced data sharing protocols among stakeholders and more advanced traceability tools, making it possible to track and verify medications in real time.
These updates are designed to improve patient safety by reducing the risk of counterfeit drugs and increasing transparency in how pharmaceuticals are distributed. To stay compliant, healthcare organizations and their partners will need to invest in advanced systems tailored for compliance and risk management.
What is the MAPS Act, and how does it improve healthcare supply chain security?
The MAPS Act aims to bolster the security and dependability of the healthcare supply chain. It establishes clear guidelines for tracking and tracing medical devices, improving transparency and minimizing the chances of counterfeit products entering the system. With its focus on stricter oversight and accountability, the Act works to safeguard against supply chain disruptions and cyber threats. This, in turn, plays a key role in protecting patient safety and maintaining trust in the healthcare system.
How can healthcare organizations strengthen cybersecurity while ensuring secure data sharing?
Healthcare organizations can bolster their cybersecurity defenses and ensure safer data sharing by using specialized risk management tools designed for the healthcare sector. For example, platforms like Censinet RiskOps™ streamline third-party risk evaluations, help measure cybersecurity performance, and promote collaboration in managing risks effectively.
To protect sensitive data, it's essential to create robust cybersecurity plans, implement secure data-sharing practices, and embed risk management tools into daily operations. Fostering an environment of ongoing risk evaluation and adhering to industry standards are also critical for maintaining data security and compliance.


