CLIA Laboratory Vendor Compliance: Third-Party Risk for Diagnostic Services
Post Summary
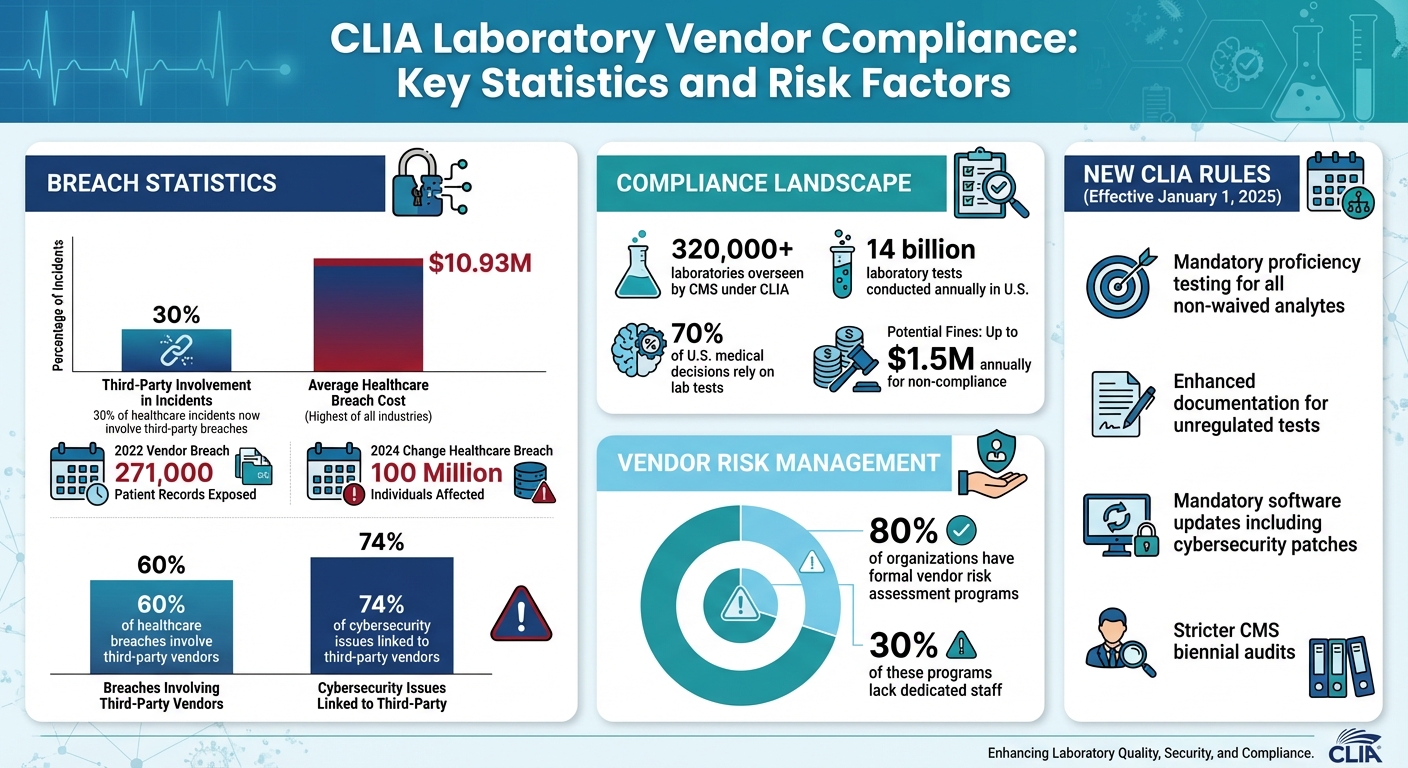
Healthcare organizations depend on CLIA-certified labs for accurate diagnostic testing, but this reliance introduces risks like data breaches, compliance failures, and financial penalties. For example, a 2022 breach exposed 271,000 patient records due to vendor mishandling. With stricter CLIA rules in place since January 2025, labs face tighter oversight on testing, cybersecurity, and record-keeping.
Key takeaways:
- Data breaches: Third-party breaches now account for 30% of incidents, with average healthcare breach costs reaching $10.93M.
- Compliance risks: Missing Business Associate Agreements (BAAs) or outdated software can lead to fines up to $1.5M annually.
- New CLIA rules: Labs must maintain detailed records, comply with cybersecurity updates, and meet stricter testing standards.
- Risk management: Tools like automated assessments, cybersecurity benchmarks, and continuous monitoring are critical for reducing vendor risks.
Healthcare organizations must prioritize vendor oversight to protect patient data, meet regulatory standards, and avoid costly disruptions.
CLIA Laboratory Vendor Compliance: Key Statistics and Risk Factors
Third-Party Risks from CLIA Laboratory Vendors
CLIA Regulations Explained
The Clinical Laboratory Improvement Amendments (CLIA) establish federal quality standards to ensure diagnostic tests are accurate and reliable. These rules cover everything from personnel qualifications to quality control and proficiency testing. The most recent update to the electronic Code of Federal Regulations for CLIA (Title 42, Part 493) was made on December 4, 2025[2].
Starting January 1, 2025, CLIA introduced stricter rules that impact how healthcare organizations work with laboratory vendors. These updates require mandatory proficiency testing for all non-waived analytes, enhanced documentation for unregulated tests, and prohibit labs from forwarding proficiency testing samples or discussing them with outside parties. Labs are also required to follow manufacturer guidelines for all software updates, including cybersecurity patches, extending their responsibility to vendor-provided software. Additionally, labs must maintain detailed records of proficiency testing participation, results, and corrective actions. These records are subject to stricter CMS audits during biennial inspections, along with tougher enforcement measures[9]. These changes highlight the importance of closely monitoring laboratory vendors to ensure compliance.
Common Risks in Diagnostic Services
While CLIA provides a regulatory framework, healthcare organizations still face practical challenges when working with laboratory vendors. A lack of proper oversight can leave organizations vulnerable to data breaches, as vendors often need access to sensitive patient information[6].
As Balbix notes:
"Vendors often need access to your sensitive information, which creates weak points for potential data breaches. To lower these risks, tighten data access controls and review vendor practices." [6]
One major risk is outdated software. For example, operating systems like Windows 10, which will no longer be supported after October 14, 2025, leave labs open to malware and cyberattacks. Many diagnostic labs still rely on equipment running outdated software, increasing their risk exposure[5].
Inadequate oversight of vendors can also hinder a lab's ability to meet CLIA standards. This includes requirements for personnel qualifications, quality control, proficiency testing, and record-keeping[1]. Human errors - such as mistakes in operations, documentation issues, or unclear responsibilities - can be amplified by poor vendor management[8]. Without consistent monitoring of vendor practices and security measures, critical vulnerabilities may go unnoticed, leading to compliance failures and heightened risks[6].
Failing to include compliance requirements in vendor contracts or conduct regular audits further increases the likelihood of non-compliance with HIPAA and CLIA. High-profile breaches, like those involving SolarWinds and Kaseya, serve as cautionary tales about the dangers of insufficient vendor oversight[6].
CLIA Compliance Requirements for Laboratory Vendors
Required CLIA Standards
In the U.S., any facility conducting tests on human specimens for health assessment or disease-related purposes must comply with CLIA regulations. The Centers for Medicare & Medicaid Services (CMS) oversees more than 320,000 laboratories under these rules, ensuring test results are accurate, reliable, and delivered promptly[10][1][11].
When partnering with laboratory vendors, healthcare organizations need to confirm that these vendors adhere to specific CLIA standards. The requirements differ based on the complexity of the tests being performed, with stricter guidelines for more advanced testing. It's essential to request documentation such as personnel qualifications, proficiency testing records, training logs, and procedure manuals[15][1].
Vendors are also expected to maintain detailed records, including quality control reports, calibration logs, maintenance records, test requisitions, patient result audits, and reagent lot tracking[15][1]. Common compliance issues in 2021 included missing documentation of employee competencies, inadequate oversight of reagent storage and testing conditions, and failure to verify test accuracy[15]. Healthcare organizations should always review the vendor's CLIA certificate, whether it’s a Certificate of Waiver, Certificate for Provider-Performed Microscopy Procedures, Certificate of Registration, Certificate of Compliance, or Certificate of Accreditation[16][17]. Additionally, ensuring patient data security is a critical part of the process.
HIPAA and Data Security Requirements
In addition to meeting CLIA standards, vendors must prioritize safeguarding patient data, as data protection is just as crucial. With approximately 70% of U.S. medical decisions relying on 14 billion laboratory tests conducted annually across 330,000 CLIA-certified labs, the volume of sensitive information handled is immense[13].
A 2014 amendment to CLIA regulations requires CLIA-certified labs to provide patients with direct access to their completed test results upon request, removing a prior HIPAA Privacy Rule exception[12][13][14]. Labs must verify patient identity, deliver results in the requested format, and respond within 30 days[13]. When working with external partners - like software developers, cloud service providers, consultants, or reference labs - who access protected health information (PHI), these entities are typically classified as Business Associates under HIPAA[13].
Healthcare organizations should ensure vendors have formal Business Associate Agreements (BAAs) and demonstrate robust data security practices. This includes conducting risk assessments, implementing access controls, encrypting data, and maintaining physical security measures[13][14]. Organizations should also request proof of secure data storage, audit trail capabilities within the vendor's Laboratory Information System (LIS) or Laboratory Information Management System (LIMS), and detailed access logs to monitor changes, data access, and specimen movement[15][1].
Challenges in Managing Third-Party Risks
Problems with Visibility and Evidence Validation
Healthcare organizations often face significant blind spots due to limited visibility into their vendor networks, especially when dealing with CLIA-certified laboratory vendors [18]. These gaps can leave organizations vulnerable to risks they may not even be aware of. Adding to the complexity, manually validating certifications and security protocols for multiple vendors is not only time-consuming but can quickly overwhelm risk management teams.
Another challenge arises with third-party testing, which often fragments patient data within the electronic medical record (EMR) system. This fragmentation delays patient care and drives up follow-up costs as healthcare teams struggle to piece together scattered data.
"The importance of robust vendor risk management, or 'VRM,' practices has been illustrated by a number of cyberattacks and data breaches that have originated from vendors and gone on to significantly impact the organizations using their services." - Syed Khan, Manager of Implementation, AuditBoard [19]
These issues underscore the pressing need for tools that can automate and scale risk assessments effectively.
Scaling Risk Assessments Across Multiple Vendors
Managing relationships with numerous vendors presents a daunting challenge for risk management teams, especially when resources are stretched thin [18]. These teams are tasked with assessing, monitoring, and documenting compliance for a wide array of laboratory vendors, each with unique testing capabilities, data systems, and security protocols.
The absence of standardized processes compounds the problem. Each vendor assessment often becomes a one-off exercise, requiring hours of customization, document collection, and manual compliance tracking. This fragmented approach is unsustainable when dealing with multiple CLIA-certified labs. It leads to inconsistent oversight, overlooked risks, and compliance gaps that could result in regulatory penalties or data breaches.
To address these challenges, organizations need risk assessment methods that are both efficient and scalable.
sbb-itb-535baee
Risk Assessment and Mitigation Methods
Standardized and Automated Risk Assessments
For CLIA-certified laboratory vendors, using standardized and automated risk assessments simplifies the evaluation of critical processes like specimen handling and quality controls [20]. Tools like standardized questionnaires and checklists ensure consistency by addressing key areas such as test systems, reagents, environmental controls, and personnel qualifications [20].
Techniques like Failure Modes and Effects Analysis (FMEA) help quantify risks by assigning a Risk Priority Number (RPN). This number, calculated based on factors like severity, likelihood of occurrence, and detectability, provides a clear way to prioritize vendors requiring immediate attention [20][21]. To ensure fair and comparable evaluations, adopting clear scoring methods - whether numeric or color-coded - is essential [3].
Interestingly, while nearly 80% of organizations have formal vendor risk assessment programs, around 30% of these programs lack dedicated staff [3]. Automated software solutions can help bridge this gap by streamlining data management, tracking, reporting, and analysis [3]. These assessments should be conducted annually or more frequently, depending on changes in vendor procedures.
In addition to structured assessments, incorporating cybersecurity benchmarks can provide valuable insights into vendor security.
Using Cybersecurity Benchmarks
Cybersecurity ratings offer quantifiable insights into vendor security, particularly for vendors managing sensitive diagnostic data [22]. These ratings have been validated against real-world attacks, showing that lower scores often indicate a higher likelihood of breaches [22]. For healthcare organizations, this data is critical for identifying high-risk vendor relationships before any incidents occur.
Standardized frameworks like ISO 27001 and NIST provide a shared language for assessing supplier security practices [23]. One major benefit of cybersecurity ratings is their ability to update as a vendor's security posture evolves [22]. This real-time monitoring helps organizations track remediation efforts and stay alert to emerging vulnerabilities.
By proactively using cybersecurity benchmarks, organizations can identify weaknesses early, reducing the chances of significant data breaches.
Continuous Monitoring and Compliance Documentation
Building on risk assessments and cybersecurity benchmarks, continuous monitoring strengthens long-term vendor compliance efforts.
"Risk management constitutes an essential component of the Quality Management System (QMS) of medical laboratories." – Jayagandan Jayamani et al. [20]
Continuous monitoring is a flexible, ongoing process that tracks and documents supplier security practices to drive improvement over time [7][20][23]. This involves identifying, quantifying, prioritizing, mitigating, and continuously reviewing risks through quality assurance activities [7][20].
Compliance documentation plays a crucial role in demonstrating regulatory adherence, simplifying audits, and ensuring traceability of data, samples, and results [24][25]. A strong Quality Management System ensures these processes are standardized, protecting the integrity of lab results while keeping organizations prepared for audits. As outlined in ISO 15189:2012, laboratories are required to "evaluate the impact of work processes and potential failures on examination results as they affect patient safety and shall modify processes to reduce or eliminate the identified risks and document decisions and actions taken" [20].
Effective continuous monitoring goes beyond just maintaining records. It incorporates feedback loops that allow for ongoing adjustments based on results, turning compliance documentation into a proactive tool for identifying and addressing vulnerabilities as they arise [22]. This approach ensures that risk management evolves alongside emerging threats, keeping organizations resilient and prepared.
Censinet RiskOps™ for CLIA Vendor Compliance

Censinet RiskOps™ delivers a streamlined solution for healthcare organizations navigating the complexities of managing third-party risks, particularly for CLIA-certified laboratory vendors. By centralizing risk management and offering real-time insights into vendor compliance, the platform helps organizations address healthcare compliance challenges while maintaining scalability across multiple vendor relationships.
Features of Censinet RiskOps™
Censinet RiskOps™ simplifies the vendor risk assessment process with automated workflows designed to reduce manual effort and accelerate evaluations. Its integrated AI technology enables vendors to quickly complete security questionnaires, summarize documentation, and identify key details about product integrations and potential fourth-party risks.
The platform’s AI-powered evidence validation blends automated processes with human oversight, guided by clear rules. This ensures efficiency without compromising accuracy. Risk teams can verify vendor compliance documentation, assess HIPAA security controls, and evaluate overall cybersecurity measures with speed and precision.
A centralized command center offers healthcare organizations an intuitive dashboard that consolidates real-time data across all vendor relationships. This dashboard not only visualizes risk but also directs critical findings and tasks to the appropriate stakeholders for timely action. For CLIA-certified laboratory vendors, this means compliance data is easily accessible and well-organized.
Additionally, the platform includes continuous monitoring that keeps vendor compliance indicators updated in real time. This eliminates the need for periodic reassessments, allowing healthcare organizations to quickly detect vulnerabilities and confirm the effectiveness of remediation efforts without starting assessments from scratch.
These features work together to give healthcare organizations the tools they need to adapt their risk management strategies effectively.
Plans for Healthcare Organizations
Censinet offers three tailored plans to meet the diverse needs and resources of healthcare organizations:
- Platform Plan: Provides full access to Censinet RiskOps™ software, ideal for organizations that prefer to manage vendor risk assessments with their in-house teams.
- Hybrid Mix Plan: Combines software access with managed services, enabling organizations to use Censinet’s expertise for specific tasks while retaining control over other processes. This approach enhances risk management without requiring additional internal resources.
- Managed Services Plan: Offers fully outsourced vendor risk management, with Censinet taking care of the entire assessment process. This plan is perfect for organizations with limited staff or expertise, providing immediate access to specialized support.
All plans include access to Censinet Connect™, a collaborative risk network that streamlines vendor assessments and promotes efficient information sharing between healthcare organizations and CLIA-certified laboratory vendors.
Conclusion
Managing risks with CLIA-certified laboratory vendors requires more than occasional check-ins. Continuous oversight is essential, as lapses in monitoring can lead to serious threats to patient safety and data security. A stark example is the 2024 Change Healthcare breach, which exposed data for 100 million individuals and disrupted critical services like electronic prescribing and claims submission nationwide. This incident highlights how vendor vulnerabilities can have a direct and dangerous impact on patient care [27].
The numbers paint a worrying picture: nearly 60% of healthcare breaches involve third-party vendors, with 74% of cybersecurity issues linked to them. On top of that, the healthcare sector faces an average breach cost of $10.93 million, the highest across all industries [4][27]. These statistics underline the pressing need for a forward-thinking risk management strategy.
But this isn’t just about meeting compliance requirements. A proactive approach ensures patient data remains secure, operations stay on track, and disruptions to care delivery are minimized. To tackle these risks effectively, healthcare organizations should focus on centralized vendor data management, continuous compliance checks, and regular risk evaluations [26]. These steps align with the article's key takeaway: consistent and proactive vendor oversight is vital for protecting patient care.
Tools like Censinet RiskOps™ simplify this process by automating risk assessments, using AI to validate vendor compliance, and offering real-time updates on vulnerabilities and remediation efforts. The platform’s continuous monitoring approach eliminates redundant reassessments and provides organizations with a clear view of their risk landscape.
Whether handling vendor risks in-house, using software and expert support, or outsourcing entirely, healthcare organizations can tailor their strategies to fit their resources and needs. The ultimate goal remains the same: safeguarding sensitive healthcare data and ensuring that CLIA-certified laboratory vendors meet the necessary regulatory and cybersecurity standards to keep patients safe. This reinforces the article’s central message: ongoing monitoring and automated risk management are critical for securing vendor relationships.
FAQs
What changes to CLIA rules for laboratory vendors are coming in 2025?
Starting in 2025, laboratories will need to transition to electronic communication for all notices, certificates, and fee coupons, in compliance with new CLIA rules. By March 1, 2026, paper mailings will no longer be an option.
This change is designed to make communication faster and more efficient while phasing out traditional mail systems. Laboratories and vendors should start preparing now by updating their systems to seamlessly manage these electronic communications.
What steps can healthcare organizations take to manage third-party risks with CLIA-certified labs?
To manage third-party risks with CLIA-certified labs effectively, healthcare organizations need a well-rounded risk management program. This involves structured risk assessments, ongoing monitoring of vendor compliance, and setting clear expectations through detailed contracts.
Some key steps include conducting regular audits, ensuring compliance with CLIA regulations and cybersecurity requirements, and offering continuous staff training. Using automation tools can simplify assessments and help track compliance, reducing administrative workload while improving risk management processes. By focusing on these practices, organizations can protect sensitive patient data, maintain regulatory compliance, and mitigate risks across supply chains and diagnostic services.
Why is cybersecurity important for CLIA-certified laboratory vendors?
Cybersecurity plays a vital role for CLIA-certified laboratory vendors, safeguarding sensitive patient information, ensuring the accuracy of diagnostic results, and meeting strict regulatory standards. Without robust protections in place, labs are at risk of data breaches, ransomware attacks, and other cyber threats that could jeopardize patient safety, tarnish reputations, and result in hefty penalties.
Key protective measures include encryption to secure data, access controls to limit who can view or modify information, and regular software updates to address vulnerabilities. These steps not only help labs stay compliant with regulations like HIPAA and CMS standards but also build trust and ensure smooth, uninterrupted operations for healthcare organizations working with laboratory vendors.


