GDPR Compliance for Healthcare Vendors: International Data Transfer Risks
Post Summary
Healthcare vendors handling EU patient data face strict GDPR rules, especially for international transfers. Here's what you need to know:
- Health Data Sensitivity: Medical records, genetic info, and biometric data are classified as high-risk under GDPR.
- Penalties: Non-compliance can lead to fines up to €20 million or 4% of global revenue.
- Cross-Border Challenges: Transferring data to non-EU countries, like the U.S., requires additional safeguards due to differing privacy laws.
- Transfer Mechanisms: Options include Standard Contractual Clauses (SCCs), Binding Corporate Rules (BCRs), or adequacy decisions. Each comes with specific requirements, like Transfer Impact Assessments (TIAs).
- Transparency: Patients must be informed about how their data is handled and the risks of international transfers.
To reduce risks, healthcare vendors should implement encryption, limit data transfers, and use automated tools like Censinet RiskOps™ for compliance management.
GDPR Requirements for International Data Transfers
How GDPR Applies to International Transfers
When it comes to cross-border data activities, GDPR adds another layer of complexity. Thanks to its extraterritorial reach under Article 3, U.S. healthcare vendors - already navigating HIPAA compliance - must also meet GDPR's stricter requirements when handling data from EU patients. Whether you're offering telemedicine services to EU residents, running clinical trials involving European participants, or managing cloud-based health records for EU healthcare providers, GDPR's rules apply. Health data, classified as a special category of personal data under Article 9, is subject to strict limitations. Its processing is generally prohibited unless it meets specific conditions, and these restrictions only tighten when data crosses international borders.
To comply, organizations need a solid legal framework and approved mechanisms for data transfers.
Legal Basis and Transfer Mechanisms
International data transfers under GDPR require a two-step approach. First, you need a legal basis under Articles 6 and 9, such as explicit patient consent, contractual necessity, legal obligations, or public interest. Second, you must implement an approved transfer mechanism under Chapter V.
One option is the EU-U.S. Data Privacy Framework (DPF), adopted by the European Commission in July 2023. However, its future remains uncertain, as it faced a legal challenge in the Court of Justice of the European Union just two months after its adoption [3].
For many, Standard Contractual Clauses (SCCs) or Binding Corporate Rules (BCRs) are the go-to mechanisms. But these come with their own requirements, such as conducting a Transfer Impact Assessment (TIA). A TIA evaluates whether the destination country's laws - like U.S. surveillance regulations - undermine GDPR protections. If risks are identified, supplementary measures must be implemented [5].
Once the legal groundwork is set, the focus shifts to ensuring transparency and safeguarding patients' rights.
Transparency and Data Subject Rights
Under GDPR Articles 13 and 14, patients must be informed about international data transfers and the risks involved. This means providing clear, accessible details about how their data is handled across borders. If explicit consent is the legal basis, patients need to be fully informed about potential risks, especially if the destination country has weaker data protection laws or lacks independent oversight.
Transparency also requires specifying the recipient country (or countries), the legal basis for the transfer, and any known risks tied to the recipient's legal environment [7]. In cases where broad consent is used for research involving transfers to countries with lower protection standards, organizations must implement robust consent management systems. These systems should provide regular updates and make it easy for patients to withdraw their consent at any time.
Clear communication and strong consent practices are key to maintaining trust while meeting GDPR's stringent requirements.
Approved Mechanisms for GDPR-Compliant Data Transfers
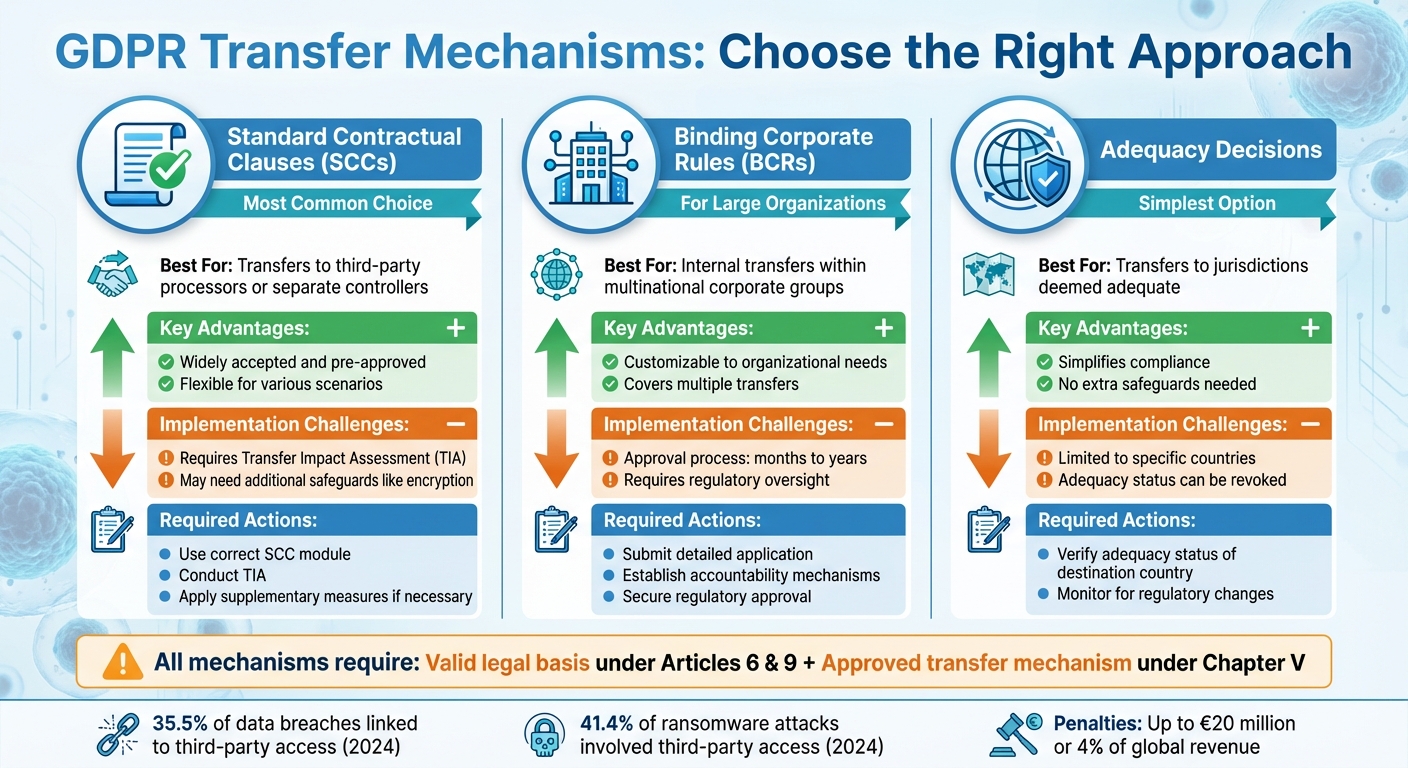
GDPR International Data Transfer Mechanisms Comparison for Healthcare Vendors
Standard Contractual Clauses (SCCs)
Standard Contractual Clauses (SCCs) are pre-approved agreements issued by the European Commission to ensure data protection obligations are upheld when transferring data outside the EU. These clauses are commonly used for sharing patient data with third-party processors, like cloud storage providers or analytics platforms, operating in non-EU countries.
The clauses themselves cannot be altered. On June 4, 2021, they were updated to include a modular format covering different transfer scenarios: Controller-to-Controller, Controller-to-Processor, Processor-to-Processor, and Processor-to-Controller. Organizations using SCCs must also conduct a Transfer Impact Assessment (TIA) to evaluate whether the recipient country’s legal framework could compromise data protection standards. If potential risks are identified, additional technical measures - such as encryption or pseudonymization - must be applied, as relying on explicit consent is not a suitable fallback option [8].
In March 2025, First Advantage, a global background screening company, confirmed its reliance on SCCs and the International Data Transfer Addendum to the EU Commission’s SCCs for cross-border data transfers with suppliers, affiliates, and customers [8].
For UK GDPR compliance, healthcare vendors should use the International Data Transfer Agreement (IDTA) or the Addendum to the EU SCCs. For instance, in May 2025, the UK Information Commissioner’s Office shared an example where a UK travel company and an Australian hotel - both acting as separate controllers - used an IDTA to transfer customer booking details. The UK company also conducted a transfer risk assessment as part of the process [6].
For intra-group data transfers, organizations might find another mechanism, outlined below, more suitable.
Binding Corporate Rules (BCRs)
Binding Corporate Rules (BCRs) are policies designed for multinational organizations to manage internal data transfers within their corporate structure. Unlike SCCs, which address transfers between separate entities, BCRs streamline data sharing within the same corporate group, such as between a U.S.-based healthcare vendor's headquarters and its European branches.
While BCRs allow organizations to adapt data protection practices to their operational needs, they require approval from the relevant supervisory authority. This approval process can be lengthy, often taking several months or even years [8].
Adequacy Decisions and Derogations
When SCCs or BCRs are not applicable, other legal frameworks may come into play. The European Commission has issued adequacy decisions for certain countries, regions, or sectors, confirming that their data protection standards are equivalent to those in the EU [3]. For transfers to these regions, additional safeguards are generally unnecessary. However, healthcare vendors must confirm whether their transfers are governed by EU GDPR or UK GDPR, as each framework has distinct adequacy requirements [6].
If no adequacy decision exists and neither SCCs nor BCRs are feasible, derogations provide limited alternatives. For instance, explicit patient consent may be used for specific, occasional transfers of sensitive health data, but it cannot be relied upon for routine data flows. Other derogations include transfers necessary for public interest purposes or to protect vital interests when a patient cannot provide consent.
How to Reduce International Data Transfer Risks
Vendor Risk Management Best Practices
Healthcare vendors must follow a two-step process for every third-country data transfer. First, ensure the processing has a valid legal basis under Articles 6 and 9 of GDPR. Second, use an approved transfer mechanism as outlined in Chapter V [5]. Both steps are essential for compliant international data transfers.
When using Standard Contractual Clauses (SCCs) or Binding Corporate Rules (BCRs), they must be accompanied by Transfer Impact Assessments (TIAs). These assessments determine whether the recipient country’s laws could compromise data protection - especially concerning government surveillance or unauthorized access [1][5][9]. If risks are identified, additional measures like encryption or pseudonymization must be implemented before proceeding with the transfer.
Maintaining an inventory of patient data flows is crucial. This documentation helps identify vulnerabilities and ensures each transfer is properly safeguarded. Strong technical measures further support these risk management efforts.
Technical Safeguards for Data Transfers
Legal mechanisms alone aren’t enough - technical measures play a key role in protecting sensitive data. Encryption and pseudonymization are essential when TIAs reveal that local laws in the recipient country may not adequately protect the data [1][9][6][4]. These techniques ensure that even if data is accessed improperly, it remains secure.
Minimizing the amount of data transferred is another critical step. Collect only the health data needed for a specific purpose, use it strictly for that purpose, and set clear retention timelines [1][2]. By transferring less data, you reduce the risk of exposure. Additionally, strict access controls should be enforced to limit who can view or process the transferred data.
The numbers speak volumes: in 2024, 35.5% of data breaches were linked to third-party access, with IT services, cloud platforms, and software vendors being the most targeted. File transfer software vulnerabilities were particularly exploited, while 41.4% of ransomware attacks involved third-party access [10]. These statistics highlight the importance of implementing robust technical safeguards.
Comparison of GDPR Transfer Mechanisms
| Mechanism | Best For | Key Advantages | Implementation Challenges | Required Actions |
|---|---|---|---|---|
| Standard Contractual Clauses (SCCs) | Transfers to third-party processors or separate controllers | Widely accepted and pre-approved | Requires Transfer Impact Assessment (TIA); may need additional safeguards like encryption | Use the correct SCC module; conduct TIA; apply supplementary measures if necessary |
| Binding Corporate Rules (BCRs) | Internal transfers within multinational corporate groups | Customizable and covers multiple transfers | Approval process can take months or years; requires regulatory oversight | Submit a detailed application; establish accountability mechanisms; secure regulatory approval |
| Adequacy Decisions | Transfers to jurisdictions deemed adequate | Simplifies compliance, no extra safeguards needed | Limited to specific countries; adequacy status can be revoked | Verify adequacy status of destination country; monitor for regulatory changes |
This table provides a clear overview to help you choose the most appropriate transfer mechanism before addressing technical and legal compliance requirements.
Regulators are ramping up enforcement on international transfers, showing less tolerance for insufficient safeguards [10]. In April 2025, the U.S. Department of Justice introduced a rule under Executive Order 14117, imposing strict limits on outbound transfers of sensitive personal data - such as health data - to "countries of concern." This new regulation requires organizations to reevaluate contracts, vendors, and internal data flows with a focus on national security [10].
sbb-itb-535baee
Using Censinet RiskOps™ for GDPR Compliance
Navigating GDPR compliance for international data transfers can be daunting, especially for healthcare vendors managing sensitive patient information. Tools like Censinet RiskOps™ and Censinet AI™ simplify this process, offering solutions to tackle complex compliance challenges with ease.
Automating Third-Party Risk Assessments
Keeping track of multiple vendors and maintaining thorough documentation is a core part of GDPR compliance. Censinet RiskOps™ takes the hassle out of these tasks by automating third-party risk assessments, making it easier for healthcare vendors to stay on top of their compliance obligations.
Censinet AI™ enhances this process by summarizing vendor evidence, highlighting critical integration details, and identifying fourth-party risks. It also generates clear, concise risk summary reports, helping organizations quickly address potential issues. Together, these tools lay a solid foundation for better management of data flows and risk reduction.
Improving Data Flow Visibility and Collaboration
Understanding and managing how patient data moves across vendors and jurisdictions is another major compliance hurdle. Censinet RiskOps™ addresses this by centralizing data flow tracking on a user-friendly dashboard. This makes it easier to spot vulnerabilities and strengthen data protection measures.
The platform also ensures that key findings from assessments are shared with the right stakeholders, enabling faster issue resolution and ongoing oversight. With centralized visibility and automation working hand-in-hand, compliance processes become far more efficient.
Accelerating Compliance with Censinet AI™
Censinet AI™ takes automation to the next level by integrating human-guided processes into critical steps like evidence validation, policy creation, and risk mitigation. This approach allows healthcare organizations to scale their risk management efforts while keeping expert oversight where it matters most. By automating routine tasks and reserving human judgment for complex decisions, the platform helps organizations meet GDPR standards more efficiently.
Conclusion
For healthcare vendors, adhering to GDPR requirements for international data transfers isn't optional - it's a necessity. Protecting health data calls for stringent measures like encryption, pseudonymization, tight access controls, regular audits, and thorough vulnerability assessments.
When transferring patient data across borders, vendors must rely on approved safeguards such as Standard Contractual Clauses (SCCs), Binding Corporate Rules (BCRs), or adequacy decisions. Additionally, conducting Transfer Impact Assessments has become crucial, particularly in light of the Schrems II ruling, to pinpoint and address potential risks before they lead to breaches or regulatory issues.
Handling these intricate demands manually can quickly overwhelm even the most capable teams. Tools like Censinet RiskOps™ and Censinet AI™ simplify the process by automating third-party risk assessments, providing centralized visibility into data flows, and streamlining evidence validation and policy creation. This allows organizations to scale their risk management efforts efficiently while benefiting from expert oversight.
FAQs
What are the main GDPR rules for transferring healthcare data internationally?
When transferring healthcare data internationally under GDPR, it’s crucial to ensure the data is properly safeguarded. This can be done using tools like Standard Contractual Clauses (SCCs) or Binding Corporate Rules (BCRs). Alternatively, transfers can rely on countries that have received an adequacy decision, meaning their data protection standards are deemed strong enough. Additionally, there must be a valid legal basis for the transfer, such as explicit patient consent or the necessity to provide healthcare services.
Healthcare vendors should also perform transfer impact assessments to identify potential risks. These assessments ensure transparency about where the data is being sent and help determine if additional safeguards are needed. By taking these steps, organizations can stay compliant while protecting sensitive health information during international data transfers.
What steps can healthcare vendors take to safely transfer patient data outside the EU while staying GDPR compliant?
Healthcare providers can safeguard patient data during international transfers by utilizing Standard Contractual Clauses (SCCs) or Binding Corporate Rules (BCRs) to align with GDPR regulations. Strengthening security involves employing tools like encryption, pseudonymization, and access controls to protect sensitive information effectively.
Another key step is conducting Transfer Impact Assessments (TIAs) to evaluate potential risks, such as unauthorized access by foreign governments, while ensuring compliance with GDPR requirements. Partnering with GDPR-compliant organizations and keeping patients informed about how their data is managed further minimizes risks and fosters trust.
How does Censinet RiskOps™ help healthcare vendors stay GDPR compliant during international data transfers?
Censinet RiskOps™ helps healthcare vendors navigate GDPR compliance by simplifying risk assessments, automating the oversight of data transfer safeguards, and keeping a close eye on data protection measures. These capabilities work together to pinpoint and address risks tied to international data transfers, ensuring alignment with GDPR requirements.
With tools that assist in implementing mechanisms like Standard Contractual Clauses or Binding Corporate Rules, Censinet RiskOps™ takes the complexity out of regulatory demands. This allows healthcare vendors to prioritize safeguarding sensitive data and staying compliant with greater ease and assurance.


