Laboratory Vendor Risk Management: Ensuring Accurate Results and Patient Safety
Post Summary
Laboratory vendor risk management involves identifying, assessing, and mitigating risks associated with third-party vendors to ensure accurate test results and patient safety.
It ensures reliable test results, prevents errors, protects patient safety, and maintains compliance with healthcare regulations.
Risks include data security breaches, supply chain disruptions, equipment failures, and noncompliance with regulatory standards.
By conducting thorough vendor assessments, implementing quality control plans, adhering to ISO standards, and leveraging tools like Censinet RiskOps™.
Compliance ensures adherence to standards like ISO 15189 and HIPAA, reducing legal risks and protecting patient safety.
It safeguards patient care, reduces financial losses, ensures regulatory compliance, and enhances trust in healthcare services.
Vendor risk management in laboratories is critical for maintaining diagnostic accuracy and protecting patient safety. Labs depend on external vendors for essential tools like diagnostic instruments, software, and reagents, but these partnerships come with risks. Cyberattacks, supply chain delays, and non-compliance with regulations can disrupt lab operations and compromise patient care.
Here’s what you need to know:
- Risks to Address: Cybersecurity threats, operational disruptions, and regulatory non-compliance are the primary concerns.
- Key Practices: Assess vendors before contracts, monitor their performance regularly, and have clear incident response plans.
- Regulatory Requirements: Labs must ensure vendors comply with HIPAA, CLIA, and other healthcare regulations.
- Tools to Help: Platforms like Censinet RiskOps™ simplify vendor risk management with centralized oversight, automated assessments, and AI-driven tools.
Main Risks from Laboratory Vendors
When working with laboratory vendors, there are three major risk areas that demand attention: cybersecurity, operational continuity, and compliance. Addressing these risks is essential to maintaining diagnostic precision and safeguarding clinical care.
Cybersecurity and Data Privacy Risks
Healthcare systems, including laboratories, digital health providers, and telehealth platforms, have become prime targets for cybercriminals. Ransomware attacks, phishing schemes, and poorly secured cloud systems have already wreaked havoc on healthcare operations [1]. For example, ransomware has forced emergency services to shut down, phishing emails have breached patient portals, and cloud misconfigurations have exposed thousands of health records in mere minutes [1].
For laboratories, a cyber incident involving a vendor can be devastating. It might block access to critical test results or compromise sensitive patient data, delaying diagnoses that could be life-saving. These risks go beyond inconvenience - they directly affect the lab's ability to function accurately and efficiently.
Operational and Continuity Risks
Service interruptions caused by vendors can disrupt laboratory workflows, leading to delays that affect patient care. As Case IQ highlights, unreliable vendors can cost organizations money, customers, and even their reputation [2].
Labs must assess two key operational risks when evaluating vendors:
- Day-to-day operational risk: The potential for a vendor's policies or practices to disrupt normal lab functions.
- Replacement risk: The difficulty of finding a suitable alternative if the vendor fails to deliver [2].
From delayed reagent deliveries to outages at reference labs, these interruptions can directly impact diagnostic timelines and patient care decisions.
Clinical Quality and Compliance Risks
Vendors are required to meet strict healthcare regulations, such as HIPAA for data privacy and CLIA for quality standards. When vendors fail to comply, they expose laboratories to regulatory penalties and jeopardize diagnostic accuracy. This not only risks patient safety but also undermines the trust placed in laboratory services.
Regulatory Frameworks and Best Practices for Laboratory Vendor Risk Management
Healthcare Regulations and Standards for Laboratory Vendors
Laboratories working with vendors who handle Protected Health Information (PHI) must ensure those vendors sign a Business Associate Agreement (BAA) and comply with key regulations like HIPAA, HITECH, and CLIA. Additionally, 21 CFR Part 11 requires stringent controls for managing electronic records [3]. To bolster security further, labs should follow the Cybersecurity and Infrastructure Security Agency's (CISA) guidance on ICT supply chain risk management, which includes using its Vendor Supply Chain Risk Management Templates [4]. Together, these regulations create a framework for implementing vendor risk controls that protect both compliance and patient care.
Practical Vendor Risk Controls
Using these regulatory guidelines as a foundation, laboratories can implement meaningful vendor controls. For instance, vendors handling PHI should encrypt this data both during transmission and while stored. Contracts with vendors must clearly outline where and how data will be stored [2]. Access controls are another critical element, and these should follow the principle of least privilege - allowing vendors access only to the systems and data they need for their specific tasks.
Monitoring vendor activities is essential, as their practices and security measures can evolve over time. Regular security assessments, annual audits, and real-time tracking of vendor access are all effective ways to identify and address emerging risks.
Classifying vendors by risk level is another smart approach. Factors like the sensitivity of the data they handle, the criticality of their services, and the potential impact of a failure should determine how much oversight they require [5]. Vendors considered high-risk should undergo more frequent audits and face stricter review standards [6]. Additionally, laboratories need to prepare for disruptions by including vendor-related scenarios in their incident response plans. These plans should define roles, responsibilities, and backup procedures to ensure operations continue smoothly during any interruptions. Making compliance a contractual obligation, reinforced by regular verification checks, ensures vendors maintain robust security practices throughout the partnership [5].
Managing Laboratory Vendor Risk Throughout the Vendor Lifecycle
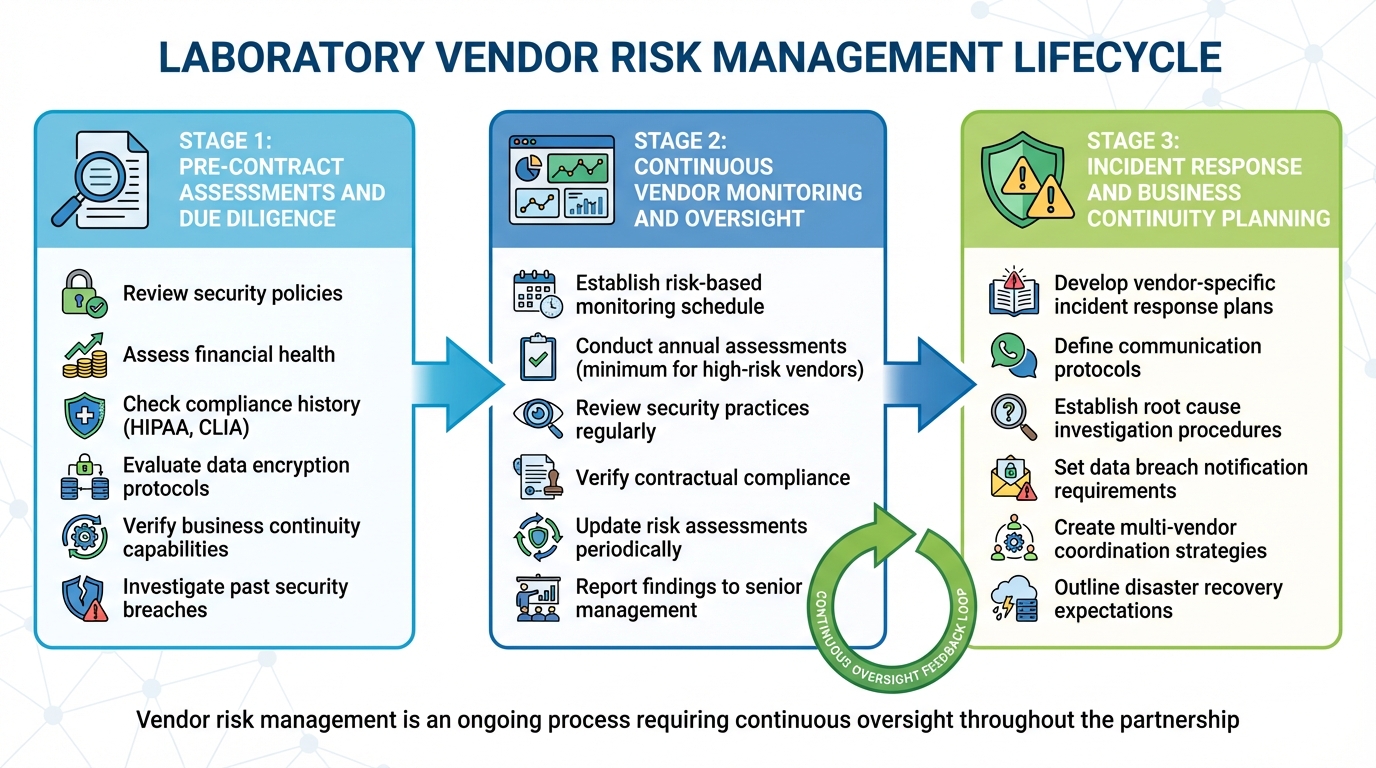
Laboratory Vendor Risk Management Lifecycle: From Assessment to Incident Response
Effective vendor risk management isn’t a one-and-done task - it’s an ongoing process that spans the entire lifecycle of a vendor relationship. From the initial evaluation to long-term oversight, laboratories must ensure their vendors consistently meet security, compliance, and operational standards. This approach builds on existing regulatory frameworks, turning them into actionable steps that cover everything from vendor selection to handling incidents.
Pre-Contract Assessments and Due Diligence
Before signing any contracts, laboratories need to thoroughly assess potential vendors. This means digging into their security policies, financial health, compliance history, and any past incidents. Tools like questionnaires can help collect details about a vendor’s security measures, privacy practices, and ability to meet regulatory requirements.
Key areas to evaluate include compliance with standards like HIPAA, data encryption protocols, and business continuity capabilities. A vendor’s track record with other healthcare organizations is also a valuable indicator. Have they faced security breaches or compliance violations in the past? Taking the time to investigate these factors can help avoid partnerships with vendors who might pose risks. Once a vendor is onboarded, the focus shifts to keeping tabs on their performance.
Continuous Vendor Monitoring and Oversight
Once the relationship is underway, keeping a close eye on vendors is essential. As Kelly Vick from Venminder points out:
Establish a monitoring schedule based on each vendor's risk level [7].
Monitoring frequency should be tailored to the vendor’s role and risk level within your organization. Vendors considered high-risk or critical will naturally require more frequent reviews. Vick also suggests:
the vendor risk assessment will need to be updated periodically – we recommend at least annually if the vendor is high risk or critical [7].
Regular monitoring involves reviewing security practices, ensuring compliance with contractual obligations, and updating risk assessments as needed. Sharing these findings with senior management and the board keeps leadership informed about potential vulnerabilities. And when issues do arise, having a well-tested incident response plan can make all the difference.
Incident Response and Business Continuity Planning
Preparation is key when it comes to handling vendor-related disruptions. Mike Miller, vCISO at Appalachia Technologies, highlights the importance of having a plan in place:
Having a vendor-specific incident response plan allows organizations to respond efficiently to security breaches, limit damage, and recover from incidents while maintaining business continuity [8].
Contracts should clearly outline expectations for business continuity, disaster recovery, incident reporting timelines, and vendor liability. A solid incident response plan should include clear communication protocols with vendors, procedures for investigating and analyzing root causes, data breach notification requirements, and strategies for coordinating responses when multiple vendors are involved [8]. Being prepared ensures that when disruptions happen, laboratories can minimize the impact and keep operations running smoothly.
sbb-itb-535baee
Using Censinet for Laboratory Vendor Risk Management
Managing vendor risk in laboratory operations is no small feat. It requires a system capable of navigating the intricate demands of healthcare cybersecurity, compliance, and operational oversight. That’s where platforms like Censinet RiskOps™ step in. Designed specifically for the healthcare industry, Censinet RiskOps™ connects healthcare delivery organizations with a vast network of over 50,000 vendors and products, offering a collaborative approach to tackle vendor risk challenges head-on [9][11].
Censinet RiskOps™ Features for Laboratories
At the heart of Censinet RiskOps™ is its Command Center, a centralized hub that provides IT, supply chain, business, and clinical leaders with clear, actionable insights. This feature is especially advantageous for laboratories juggling multiple vendors, from diagnostic equipment suppliers to laboratory information systems. With this level of visibility, leaders can quickly identify and address potential risks.
The platform also offers cybersecurity benchmarking, enabling laboratories to measure their security performance against industry standards and peers. This data arms risk teams with the insights needed to advocate for necessary improvements. Terry Grogan, CISO at Tower Health, shared the impact on efficiency:
"Censinet RiskOps allowed 3 FTEs to go back to their real jobs! Now we do a lot more risk assessments with only 2 FTEs required" [9].
Another standout feature is the 1-Click Assessments™, which allows vendors to instantly share their risk posture, complete with evidence and documentation. This eliminates the need for lengthy review cycles, making the evaluation process far more efficient [10]. For laboratories still relying on spreadsheets to track vendor risks, transitioning to a centralized platform like Censinet offers immediate advantages. James Case, VP & CISO at Baptist Health, highlighted this shift:
"Not only did we get rid of spreadsheets, but we have that larger community [of hospitals] to partner and work with" [9].
The collaborative risk network built into Censinet RiskOps™ enhances efficiency by allowing laboratories to share cybersecurity and risk data. This reduces the need for repetitive assessments, saving time and resources [9][11]. Additionally, the platform’s AI-driven tools further refine and speed up the evaluation process, making risk management more effective.
AI-Powered Efficiency with Censinet AI
Censinet AI takes the platform’s capabilities a step further by automating key aspects of risk assessments. Through tools like the Censinet Connect™ Copilot, the platform simplifies security questionnaires and validates evidence, significantly reducing the time spent on vendor evaluations [9][10]. This level of automation is invaluable for laboratories needing to quickly assess potential risks.
Workflow automation is another critical feature, streamlining the entire risk assessment process with standardized questionnaires and automated end-to-end evaluations [10]. By addressing risks earlier in the process, laboratories can negotiate better contract terms, mitigate the effects of data breaches or ransomware attacks, and recover more quickly from disruptions like supply chain outages [11].
With its combination of centralized tools and AI-powered automation, Censinet RiskOps™ offers laboratories a comprehensive solution to manage vendor risks efficiently and effectively.
Conclusion: Building Better Laboratory Vendor Risk Management
Effective laboratory vendor risk management is essential for ensuring patient safety and maintaining the accuracy of diagnostic results. Since medical decisions often rely heavily on laboratory test outcomes, any unmanaged vendor risk can disrupt the entire healthcare delivery process [12][13].
To address these challenges, laboratories must have a clear, centralized view of their vendor ecosystem. This means understanding factors like cybersecurity practices, operational stability, compliance with regulations, and the potential impact on clinical quality. A unified platform can bring all these elements into focus, offering the visibility and control needed.
Censinet RiskOps™ provides a tailored solution for the healthcare sector. With tools like the Command Center, 1-Click Assessments™, and a collaborative risk network powered by AI automation, this platform enables laboratories to identify, evaluate, and mitigate vendor risks effectively. It streamlines the process, reducing resource demands while maintaining thorough oversight.
Shifting to a proactive approach in vendor risk management allows laboratories to monitor risks continuously. By addressing potential issues early, they can secure better contracts, avoid data breaches, and ensure seamless operations. This proactive stance not only protects the integrity of test results but also prioritizes patient well-being at every step.
FAQs
What steps can laboratories take to ensure their vendors meet healthcare regulations like HIPAA and CLIA?
Laboratories can safeguard compliance with healthcare regulations like HIPAA and CLIA by establishing a strong vendor risk management process. This involves several key actions, starting with signing Business Associate Agreements (BAAs) with vendors who handle Protected Health Information (PHI). Regular risk assessments and consistent monitoring of vendor security practices are also essential to confirm they meet the necessary standards.
To further strengthen compliance, laboratories should develop clear internal policies and consider using specialized tools to automate compliance tracking. Including well-defined terms in vendor contracts can provide additional safeguards. Lastly, ongoing staff training plays a crucial role in ensuring that everyone involved understands their responsibilities and actively supports compliance initiatives.
What are the essential steps to assess and manage cybersecurity risks with laboratory vendors?
To tackle cybersecurity risks with laboratory vendors, it's crucial to have a well-organized vendor risk management program in place. Start by sorting vendors into categories based on their risk levels. Then, conduct in-depth security assessments to pinpoint any weaknesses. Make sure vendors meet all agreed-upon security standards outlined in contracts and keep a close eye on their cybersecurity measures over time. Consistent oversight and a solid incident response plan are essential for safeguarding sensitive data and ensuring patient safety.
How does Censinet RiskOps™ improve laboratory vendor risk management efficiency?
Censinet RiskOps™ takes the complexity out of managing laboratory vendor risks. By automating risk assessments and optimizing workflows, it provides real-time visibility into potential risks. This means labs can make quicker, more informed decisions while staying aligned with regulatory requirements.
The platform also supports continuous monitoring and swift issue resolution, cutting down the time and effort involved in managing vendor risks. Ultimately, it helps labs focus on what matters most - delivering accurate results and ensuring patient safety.
Related Blog Posts
Key Points:
What is laboratory vendor risk management?
- Definition: Laboratory vendor risk management is the process of identifying, assessing, and mitigating risks associated with third-party vendors to ensure accurate test results and patient safety.
- Focus Areas: It addresses risks in pre-analytical, analytical, and post-analytical processes, including specimen handling, equipment reliability, and result communication.
Why is vendor risk management critical in laboratories?
- Patient Safety: With nearly 70% of clinical decisions based on lab results, ensuring vendor reliability is essential to protect patient outcomes.
- Operational Efficiency: Effective risk management minimizes disruptions caused by vendor failures, such as delayed testing or equipment breakdowns.
- Regulatory Compliance: Adherence to standards like ISO 15189 and HIPAA ensures legal compliance and avoids penalties.
What are the main risks in laboratory vendor relationships?
- Data Security Risks: Cyberattacks, ransomware, and data breaches can compromise sensitive patient information.
- Supply Chain Disruptions: Material shortages, delays, and global trade issues can halt lab operations.
- Equipment Failures: Calibration errors, maintenance lapses, and outdated technology can lead to inaccurate results.
- Compliance Challenges: Noncompliance with regulations like CLIA or FDA standards can result in fines and reputational damage.
How can laboratories mitigate vendor-related risks?
- Vendor Assessments: Conduct thorough evaluations of vendor reliability, compliance history, and risk profiles.
- Quality Control Plans: Implement robust quality assurance measures to monitor vendor performance.
- Adherence to Standards: Follow ISO 15189 and ISO 31000 guidelines for risk management and quality assurance.
- Automation Tools: Use platforms like Censinet RiskOps™ to streamline risk assessments and monitor vendor compliance.
What role does compliance play in vendor risk management?
- Regulatory Adherence: Compliance with standards like ISO 15189 ensures that laboratory processes meet quality and safety benchmarks.
- Legal Protection: Adhering to HIPAA and CLIA regulations reduces the risk of legal penalties and reputational harm.
- Patient Trust: Compliance fosters trust by ensuring that laboratory services are reliable and secure.
What are the benefits of effective vendor risk management?
- Enhanced Patient Safety: Reduces errors and ensures accurate test results.
- Operational Resilience: Minimizes disruptions caused by vendor failures or supply chain issues.
- Financial Savings: Prevents costly errors, fines, and downtime.
- Improved Compliance: Ensures adherence to healthcare regulations and standards.


