Medical Device Vendor Risk Management: FDA Compliance and Patient Safety Best Practices
Post Summary
Because device vendors directly impact patient safety, FDA compliance, cybersecurity, and the reliability of clinical operations.
ISO 14971:2019, FDA QMSR (ISO 13485:2016), 21 CFR Part 803, and cybersecurity standards like UL 2900.
Quality failures, supply chain issues, cybersecurity vulnerabilities, PHI exposure, operational disruption, and vendor financial instability.
Through risk‑based categorization, review of certifications, contract security clauses, BAAs, and on‑site audits for high‑risk suppliers.
Via scheduled audits, CAPA programs, post‑market surveillance, MDR/MAUDE review, and continuous evaluation of device performance.
It automates vendor assessments, centralizes documentation, supports AI‑powered reviews, and provides real-time dashboards for risk and compliance oversight.
Medical device vendor risk management is critical for ensuring patient safety and meeting FDA compliance standards. As devices become more interconnected, they face increasing risks from cybersecurity vulnerabilities, regulatory changes, and quality issues. Here's what you need to know:
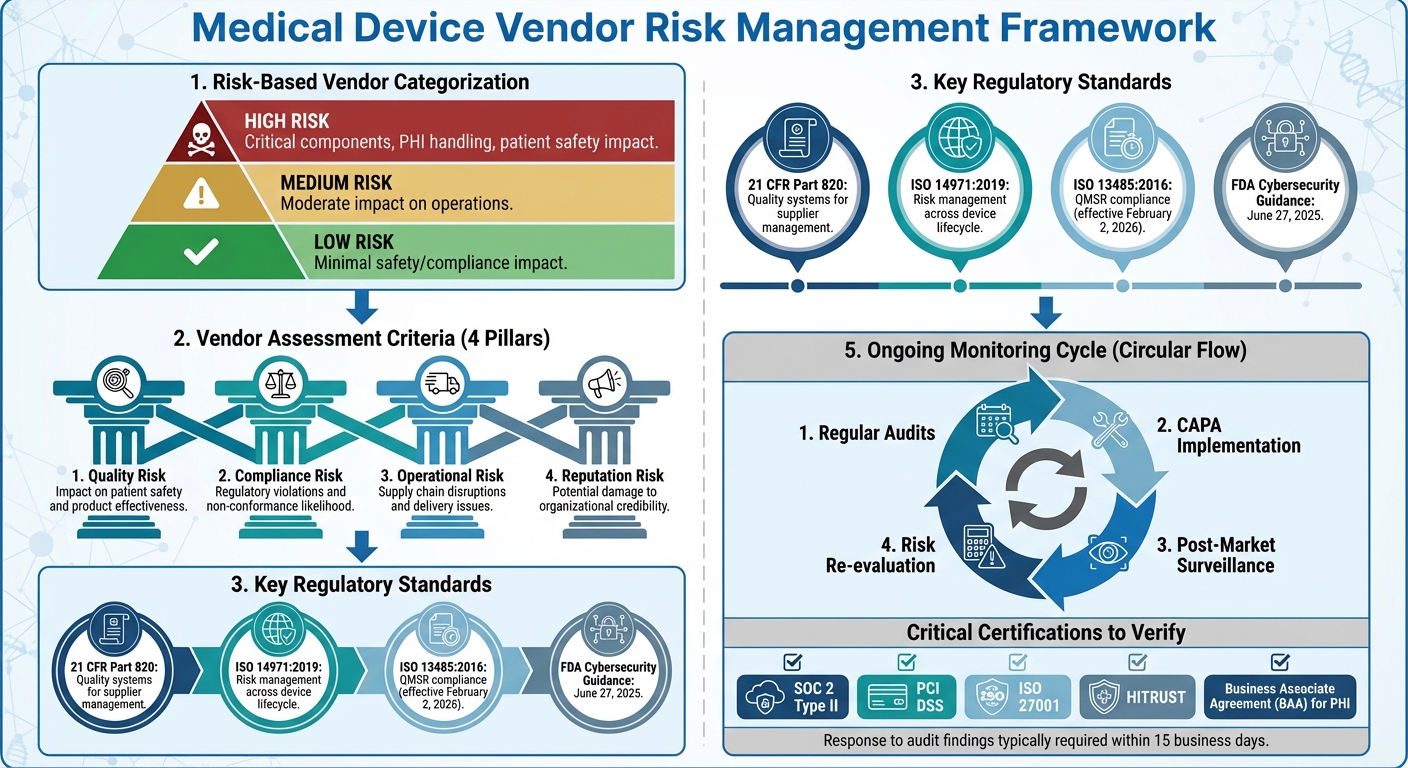
Medical Device Vendor Risk Management Framework: Assessment to Monitoring
Creating a Risk-Based Vendor Evaluation Framework
Ensuring compliance with FDA regulations and protecting patient safety requires a well-structured vendor evaluation framework. The foundation of such a framework involves categorizing suppliers based on their potential impact on patient safety and regulatory adherence. Vendors supplying critical components or handling sensitive data, like Protected Health Information (PHI), demand closer scrutiny. As ComplianceQuest aptly puts it:
"In medical device manufacturing, suppliers are more than partners, they are a direct extension of your quality system. Every component, raw material, or service provided by a supplier has the potential to impact patient safety, regulatory compliance, and brand reputation"
.
By adopting a risk-based approach, healthcare organizations can allocate their resources effectively, focusing on vendors where oversight is most critical. This involves grouping vendors into high, medium, or low-risk categories, paving the way for more precise risk assessment criteria [2]. Recent breaches underline the importance of this approach, as poor vendor evaluation can lead to serious consequences.
Defining Risk Criteria for Vendor Assessment
A supplier risk matrix is an essential tool for evaluating vendors. This matrix should assess several risk factors, including:
In medical device manufacturing, risk is often defined as the combination of the likelihood of harm occurring and the severity of that harm [5].
When conducting due diligence, healthcare organizations should review a vendor’s certifications, operational resilience, financial stability, and cybersecurity measures [6]. This includes verifying critical documents like cybersecurity certifications, insurance policies, and credit histories [7]. For vendors managing PHI, establishing a Business Associate Agreement (BAA) is crucial to ensure HIPAA compliance [7]. Additionally, organizations should assess fourth-party risks by examining the reliability of a vendor’s own suppliers and how they might affect operations [6][7].
Failing to properly screen vendors can lead to severe financial and reputational damage, as evidenced by high-profile breaches linked to third-party vulnerabilities [6].
Using ISO 14971 for Vendor Risk Management
The principles of ISO 14971:2019 - focused on identifying hazards, evaluating risks, and implementing controls - can be directly applied to vendor management [3].
Healthcare organizations can integrate these principles by performing risk analyses for each supplier relationship. This involves identifying potential hazards tied to vendor products or services, estimating the likelihood and severity of harm, and determining whether the risks are acceptable or require additional controls. These steps align seamlessly with vendor qualification and management practices.
Vendor Selection and Qualification Best Practices
For medical device manufacturers, clear criteria for vendor selection, evaluation, and re-evaluation are non-negotiable. A risk-based strategy ensures that high-risk suppliers undergo rigorous qualification processes and audits, while low-risk vendors may require lighter oversight [2]. Security qualifications and regulatory compliance should always take precedence over cost, as the fallout from breaches can far outweigh any short-term savings [7].
When selecting vendors, prioritize those with relevant certifications and robust contracts. These contracts should explicitly address data protection, confidentiality, security measures, and exit strategies [6]. Partnering with vendors certified in standards like SOC 2 Type II, PCI DSS, ISO 27001, or HITRUST can simplify due diligence and demonstrate adherence to industry standards [6][7]. For critical vendors, on-site assessments can uncover risk management gaps that might not be evident in self-reported data [6].
Many leading organizations have established comprehensive vendor security programs. For example, Microsoft’s Supplier Security and Privacy Assurance (SSPA) program requires suppliers to align with its privacy and security principles, with high-risk vendors undergoing independent compliance verification [6]. Similarly, Adobe’s Vendor Security Review Program mandates that vendors complete detailed questionnaires and provide certifications like SOC 2 Type II and ISO 27001. Medium and high-risk vendors are also subject to continuous monitoring and annual security reviews [7].
Monitoring and Managing Vendor Performance
Choosing the right vendors is just the first step. The real work begins with ongoing oversight, which ensures suppliers continue to meet regulatory standards and maintain the quality levels critical for patient safety. Without consistent monitoring, vendors risk falling out of compliance, which could have serious consequences.
Conducting Regular Vendor Audits
Regular audits are essential to verify that vendors meet both regulatory and contractual obligations. The FDA's Quality System Inspection Technique (QSIT) provides a structured framework for these audits, dividing quality system elements into major subsystems - like Management Controls, Design Controls, Corrective and Preventive Actions (CAPA), and Production and Process Controls (P&PC) - and supporting subsystems, such as facility and equipment controls.
To prepare for an audit, notify vendors in advance about the schedule, objectives, and required documentation. Before the on-site inspection, review any recalls, Medical Device Reports, or specification changes to focus the audit on key areas.
During the audit, the goal is to evaluate the vendor's quality management system, with special attention to the CAPA subsystem, which includes complaint handling and medical device reporting. Auditors gather objective evidence through inspections, testing, and record reviews to support their findings.
The FDA categorizes inspections into different levels:
Inspection Level
Type of Inspection
Focus Areas
1
Abbreviated
CAPA plus P&PC or Design Controls
2
Comprehensive
Management Controls, Design Controls, CAPA, P&PC
Special
Compliance Follow-up or For Cause
As directed by inspectional guidance
After the audit, communicate any findings to the vendor, requesting a written response - typically within 15 business days. This response should outline specific corrective actions, evidence of their implementation, and any supporting documentation. Once received, the response is evaluated to determine the next steps, which could range from minor adjustments to more serious regulatory or contractual actions.
Audit results are a key driver for corrective and preventive measures.
Implementing Corrective and Preventive Actions (CAPA)
A strong CAPA system is essential for addressing nonconformances. This process involves documenting issues, identifying their root causes, and implementing corrective actions, such as revising processes, improving quality controls, or providing additional training. The effectiveness of these measures must be verified during follow-up audits. If problems persist, escalation steps - like imposing contractual penalties or even terminating the vendor relationship - may be necessary.
In addition to immediate fixes, CAPA plays a critical role in long-term risk management through post-market surveillance.
Using Post-Market Surveillance for Risk Mitigation
Post-market surveillance ensures that device performance and emerging risks are continuously monitored, complementing FDA oversight. Tools like the Medical Device Reporting database and the Manufacturer and User Facility Device Experience (MAUDE) database help identify potential quality issues, recalls, or adverse events that could impact patient safety.
A well-functioning CAPA subsystem is central to effective post-market surveillance. Routine inspections ensure vendors leverage both pre- and post-market data to detect and address product or quality issues. By maintaining procedures for Medical Device Reporting in line with 21 CFR Part 803, vendors can take timely corrective actions, protecting public health and driving ongoing improvements in quality and safety.
sbb-itb-535baee
Using Censinet RiskOps for Vendor Risk Management

Managing vendor risks in healthcare can feel like walking a tightrope - balancing compliance, efficiency, and limited resources. Tools like Censinet RiskOps™ make this process more manageable by offering a centralized system to oversee medical device vendor risks and meet regulatory demands. For healthcare organizations, this means scaling oversight without the need to grow staff or compromise compliance standards.
Streamlining Vendor Risk Assessments with Censinet RiskOps
Censinet RiskOps™ simplifies third-party risk management by providing a single platform that handles everything from vendor onboarding to ongoing compliance checks. It automates workflows, standardizes vendor evaluation criteria, and tracks compliance automatically. The result? Administrative tasks are significantly reduced, freeing up risk teams to focus on higher-level oversight and strategy.
This streamlined approach ensures that teams can dedicate their energy to the big picture, rather than getting bogged down in repetitive tasks.
Improving Collaboration and Oversight with Censinet AI™
With Censinet AI™, vendor assessments take on a whole new level of efficiency. By combining automation with human guidance, the platform accelerates the process without losing the necessary oversight. Vendors can complete security questionnaires almost instantly, while the system compiles and highlights critical evidence - like product integration details and potential fourth-party risks.
Key findings are automatically routed to the right stakeholders, ensuring timely reviews and swift resolution of issues. This not only saves time but also strengthens collaboration across teams, making the entire process more seamless and responsive.
Scaling Risk Management in Healthcare
For healthcare organizations juggling multiple vendor relationships, scaling risk management is a constant challenge. Censinet RiskOps addresses this by acting as a central command center, delivering real-time insights into vendor risks. This makes it easier to spot trends, prioritize high-risk vendors, and allocate resources where they're needed most.
Importantly, the platform supports a balance between automation and human decision-making. Risk teams remain in control, using automation as a tool to handle the heavy lifting while ensuring that critical decisions are made with care. By aggregating data from various sources into one comprehensive view, the platform supports both everyday operations and long-term planning, all while maintaining the rigor needed to protect patients and meet regulatory requirements.
Conclusion: Building a Safer and Compliant Vendor Ecosystem
Key Takeaways for Healthcare Organizations
Creating a safer vendor ecosystem requires thoughtful planning and ongoing oversight. For healthcare organizations, aligning vendor risk management practices with ISO 13485:2016 is crucial as the FDA's Quality Management System Regulation (QMSR) takes effect on February 2, 2026. This regulation emphasizes the importance of thorough management reviews, quality audits, and supplier audit reports [4].
A good starting point is to analyze existing vendor audit records to ensure they align with QMSR requirements [4]. Additionally, incorporating ISO 14971:2019 principles into vendor evaluations can help identify and control hazards throughout a medical device's lifecycle. This approach minimizes the risk of device-related issues [3]. Equally important is the need for manufacturers and healthcare organizations to strengthen their cybersecurity measures [1].
These steps are essential for adapting to the changing landscape of vendor risk management.
The Future of Vendor Risk Management in Healthcare
The approach to vendor risk management is evolving, with a clear trend toward harmonizing regulations and increasing scrutiny. The FDA’s adoption of ISO 13485:2016 reflects a push to align U.S. standards with international guidelines, which can streamline the approval process for safe and effective medical devices [4]. However, as medical devices become more interconnected - linking to the Internet, hospital networks, and other systems - cybersecurity risks are growing. Addressing these risks requires proactive, comprehensive strategies [1].
The FDA has also made regulatory guidance more accessible and forward-thinking. For example, its final guidance, "Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions", released on June 27, 2025, highlights the importance of managing risks throughout the device lifecycle - from design to disposal [1]. Healthcare organizations that adopt this lifecycle approach will not only enhance patient safety but also stay ahead of compliance requirements. Ignoring these risks can lead to costly consequences, including direct remediation expenses, reputational damage, and higher insurance premiums [8].
FAQs
How does ISO 14971:2019 support vendor risk management for medical devices?
ISO 14971:2019 outlines a systematic method for handling risks linked to medical devices, including those arising from third-party vendors. It guides organizations in identifying, assessing, and addressing potential risks throughout a device's entire lifecycle, prioritizing both patient safety and regulatory adherence.
Incorporating risk management into processes like vendor selection, ongoing performance evaluation, and post-market activities encourages proactive strategies to minimize risks. This approach ensures alignment with FDA guidelines and global standards, supporting the development of safer and more dependable medical devices.
What should healthcare organizations do to prepare for the new QMSR regulation?
To get ready for the upcoming QMSR regulation, healthcare organizations need to align their quality management systems with ISO 13485:2016 standards. This means focusing on strong risk management practices and safeguarding electronic records and signatures.
Some key actions to take include performing a gap assessment to pinpoint areas that need improvement, training staff to understand the updated requirements, and drafting a detailed plan to complete the transition before the February 2, 2026, deadline. Taking these steps early can help ensure compliance while prioritizing patient safety.
Why is it important to continuously monitor medical device vendors to ensure patient safety?
Keeping a close eye on medical device vendors is crucial for ensuring patient safety. Regular monitoring allows organizations to spot potential risks early, confirm that vendors are meeting quality and safety standards, and minimize the likelihood of device malfunctions or safety concerns over time.
By taking a proactive approach, organizations can maintain compliance with FDA regulations and industry standards, all while protecting patients throughout the entire lifecycle of the medical device.
Related Blog Posts
- Building Vendor Risk Frameworks for Healthcare IT
- Healthcare Vendor Risk Management Framework: Templates, Tools, and Best Practices
- Medical Device Vendor Risk Management: FDA Compliance and Patient Safety Best Practices
- Surgical Equipment Vendor Risk Management: Operating Room Safety and Reliability
{"@context":"https://schema.org","@type":"FAQPage","mainEntity":[{"@type":"Question","name":"How does ISO 14971:2019 support vendor risk management for medical devices?","acceptedAnswer":{"@type":"Answer","text":"<p>ISO 14971:2019 outlines a systematic method for handling risks linked to medical devices, including those arising from third-party vendors. It guides organizations in identifying, assessing, and addressing potential risks throughout a device's entire lifecycle, prioritizing both patient safety and regulatory adherence.</p> <p>Incorporating risk management into processes like vendor selection, ongoing performance evaluation, and post-market activities encourages proactive strategies to minimize risks. This approach ensures alignment with FDA guidelines and global standards, supporting the development of safer and more dependable medical devices.</p>"}},{"@type":"Question","name":"What should healthcare organizations do to prepare for the new QMSR regulation?","acceptedAnswer":{"@type":"Answer","text":"<p>To get ready for the upcoming QMSR regulation, healthcare organizations need to align their quality management systems with <strong>ISO 13485:2016</strong> standards. This means focusing on strong risk management practices and safeguarding electronic records and signatures.</p> <p>Some key actions to take include performing a <strong>gap assessment</strong> to pinpoint areas that need improvement, training staff to understand the updated requirements, and drafting a detailed plan to complete the transition before the February 2, 2026, deadline. Taking these steps early can help ensure compliance while prioritizing patient safety.</p>"}},{"@type":"Question","name":"Why is it important to continuously monitor medical device vendors to ensure patient safety?","acceptedAnswer":{"@type":"Answer","text":"<p>Keeping a close eye on medical device vendors is crucial for ensuring patient safety. Regular monitoring allows organizations to spot potential risks early, confirm that vendors are meeting quality and safety standards, and minimize the likelihood of device malfunctions or safety concerns over time.</p> <p>By taking a proactive approach, organizations can maintain compliance with <strong>FDA regulations</strong> and industry standards, all while protecting patients throughout the entire lifecycle of the medical device.</p>"}}]}
Key Points:
Why is vendor risk management critical for medical devices?
- Vendors are part of the device quality system, impacting safety and regulatory compliance
- Cybersecurity vulnerabilities in device software can jeopardize patient health
- FDA QMSR (ISO 13485) requires supplier control and documented oversight
- Third‑party failures disrupt clinical workflows, recalls, or patient care
- PHI exposure risks require HIPAA‑aligned contracts and controls
How does a risk‑based vendor evaluation framework work?
- Categorize vendors into high, medium, and low risk
- Evaluate safety impact, data access, device criticality, and regulatory exposure
- Use supplier risk matrices assessing likelihood × severity
- Review certifications such as B'SOC 2, ISO 27001, HITRUST, PCI DSS
- Consider financial stability, cybersecurity maturity, insurance coverage
- Assess fourth‑party risks tied to vendor suppliers
How do ISO 14971 and FDA QMSR support vendor risk management?
- ISO 14971 provides hazard identification, risk evaluation, and mitigation
- QMSR aligns U.S. regulations with ISO 13485:2016, requiring supplier audits
- Encourages lifecycle risk controls, from design to post‑market
- Requires documented supplier oversight, audits, and CAPA
- Supports consistent global regulatory compliance
What are best practices for vendor selection and qualification?
- Require security certifications and audited quality programs
- Include contract clauses for data protection, breach reporting, and exit plans
- Hold on‑site audits for critical suppliers
- Ensure BAAs for vendors handling PHI
- Prioritize patient safety and compliance over cost savings
How does ongoing vendor monitoring protect patient safety?
- Conduct regular audits aligned with FDA’s QSIT/QMSR inspection models
- Review recalls, MDRs, MAUDE reports, and safety alerts
- Maintain strong CAPA processes with documented follow‑up
- Use post‑market surveillance to detect device issues early
- Enforce corrective actions and escalate when necessary
How does Censinet RiskOps™ modernize medical device vendor oversight?
- Provides centralized vendor inventories and risk profiles
- Automates risk assessments, evidence validation, and security reviews
- Uses Censinet AI™ to summarize documentation and identify fourth‑party risk
- Offers real-time dashboards for vendor performance and compliance
- Scales oversight without hiring additional staff
- Enhances collaboration across clinical, IT, supply chain, and risk teams


