Pharmacy Vendor Risk Management: Medication Safety and Supply Chain Security
Post Summary
Pharmacies rely on vendors for manufacturing, distribution, technology, and logistics to deliver medications. Any disruption - like shortages, quality issues, or cyberattacks - can directly impact patient care. Effective vendor risk management ensures medication safety, supply chain reliability, and data security. Here's what you need to know:
- Key Risks: Medication quality (e.g., contamination, counterfeits), supply chain vulnerabilities (e.g., global dependencies, shortages), and cybersecurity threats (e.g., data breaches, ransomware).
- Risk Management Steps: Establish a structured program covering vendor selection, onboarding, monitoring, and termination.
- Tools & Tech: Use platforms like Censinet for faster risk assessments, automated workflows, and real-time monitoring.
Pharmacies can reduce risks by enforcing strict quality controls, ensuring compliance with regulations (e.g., DSCSA), and integrating vendor safeguards into daily operations. Technology simplifies these processes, helping protect patient safety and maintain secure supply chains.
Main Risk Categories in Pharmacy Vendor Management
Breaking down pharmacy vendor risks into distinct categories helps focus management efforts effectively. Here, we explore three key areas: medication quality, supply chain vulnerabilities, and cybersecurity threats. Each of these risks can significantly impact patient care and pharmacy operations in unique ways.
Medication Quality and Clinical Risks
Ensuring the quality of medications is non-negotiable. The process demands strict adherence to quality standards during manufacturing. When these standards falter, patients may face exposure to contaminated, mislabeled, or ineffective drugs. As the FDA highlights:
"The U.S. drug supply chain remains one of the safest in the world. However, the drug supply chain has become increasingly complex as it reaches beyond U.S. borders. Threats to the supply chain such as counterfeiting, diversion, theft and imports of falsified, unapproved or otherwise unsafe drugs, could result in unsafe, ineffective drugs in U.S. distribution" [1].
Counterfeit drugs, often containing incorrect or harmful ingredients, pose a serious risk to patient safety [1]. Beyond counterfeiting, impurities in Active Pharmaceutical Ingredients (APIs) are another major concern. For example, losartan impurities were flagged as a significant issue. In an interview with Pharmacy Times, Vimala Raghavendran, head of the Pharmaceutical Supply Chain Center at US Pharmacopeia (USP), explained how pharmacists must act swiftly to identify other brands or National Drug Codes (NDCs) tied to the same API manufacturer to assess the potential impact on patients [2].
Supply Chain and Distribution Risks
Medication availability depends on a global network that is both intricate and fragile. Disruptions in this network can quickly ripple through the system. The USP Medicine Supply Map, which covers 92% of generic pharmaceuticals, reveals that India is the leading source for APIs used in the U.S., with China also playing a major role [2]. This reliance on a few key regions creates vulnerabilities when production issues or quality failures arise.
Another challenge is the economic pressure on drug manufacturing. Low pricing, while beneficial for consumers, can discourage production and lead to supply shortages. Raghavendran offers a potential solution:
"Identifying what is driving the vulnerability of a specific drug will then allow you to shore up the resilience of that supply chain by acting. So, for example, maybe finding an alternate supplier or creating a predictable market or maybe even raising price, and the solution will depend on the drug" [2].
Timely communication is also critical. Captain Catherine Chew, a pharmacist in the FDA's Division of Drug Information, stresses the importance of early warnings:
"Advanced notification to FDA of a potential drug shortage or disruption in supply is essential. Without early warning, FDA can't engage with the manufacturer and other stakeholders to begin to address the shortage, and maybe even prevent it" [3].
While supply chain risks remain a persistent challenge, the increasing reliance on technology introduces a new layer of complexity: cybersecurity.
Cybersecurity and Data Protection Risks
Modern pharmacies rely heavily on interconnected technologies to manage patient information, streamline operations, and facilitate services like e-prescribing. However, these technologies also create multiple entry points for cyberattacks. Ransomware can bring pharmacy operations to a standstill, while data breaches exposing sensitive patient information violate HIPAA regulations and erode trust.
The impact of a security incident isn’t limited to a single pharmacy. If a technology vendor experiences a breach, every pharmacy relying on their systems can be affected. This makes it essential for pharmacies to evaluate and ensure the cybersecurity measures of their vendors as part of a comprehensive risk management strategy.
Building a Pharmacy Vendor Risk Management Program
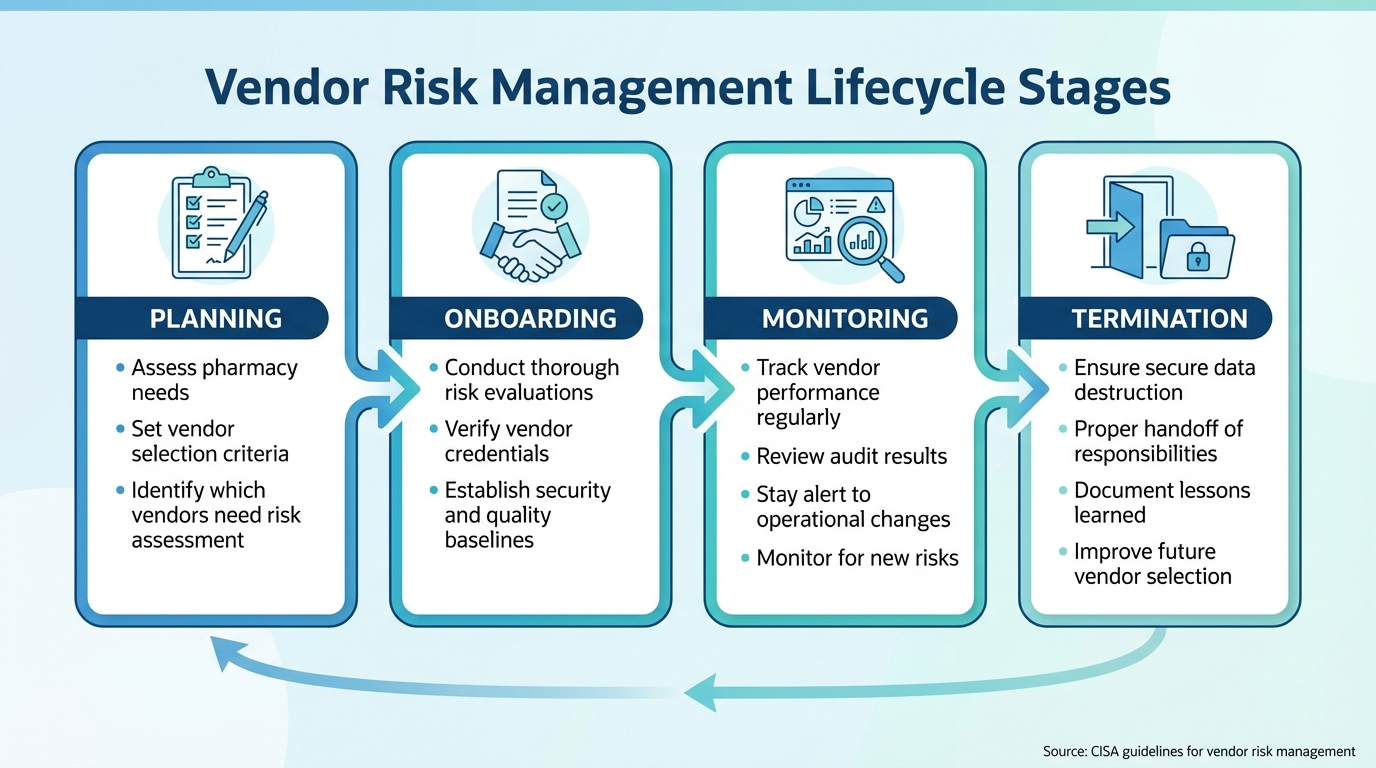
Pharmacy Vendor Risk Management Lifecycle: 4 Critical Stages
Establishing a vendor risk management program for pharmacies requires a well-defined approach that spans the entire vendor relationship - from the initial selection process to ongoing oversight and, eventually, contract termination. This structured framework ensures that risks are identified, assessed, and managed effectively.
Vendor Risk Management Lifecycle Stages
The vendor lifecycle includes four main phases: planning, onboarding, monitoring, and termination.
- Planning: This is where pharmacies assess their needs and set criteria for vendor selection. At this stage, it’s crucial to identify which vendors require a risk assessment based on the services or products they provide.
- Onboarding: Before starting any business relationship, pharmacies must conduct thorough risk evaluations, verify credentials, and establish baseline standards for security and quality.
- Monitoring: Once a vendor is active, regular oversight is key. This includes tracking their performance, reviewing audit results, and staying alert to changes in the vendor’s operations or ownership that could introduce new risks. The Cybersecurity and Infrastructure Security Agency (CISA) emphasizes the importance of vigilance in an ever-changing threat environment [5].
- Termination: When the relationship ends, focus on secure data destruction, proper handoff of responsibilities, and documenting lessons learned to improve future vendor selections.
This lifecycle approach helps pharmacies apply consistent and rigorous quality risk principles, ultimately protecting medication safety and patient care.
Applying Quality Risk Management Principles
Quality risk management centers on identifying, evaluating, and controlling risks that could impact medication safety and patient outcomes. This involves assessing potential failures, their likelihood, and their severity.
For example, a vendor supplying critical medications carries a much greater risk than one providing office supplies, even if both have similar cybersecurity practices. By weighing both the probability and severity of risks, pharmacies can prioritize their oversight efforts. High-risk vendors may require more stringent controls, while lower-risk relationships can be managed with less intensive measures. This risk-based approach ensures resources are allocated where they’re needed most.
Adding Risk Controls to Contracts and SLAs
Contracts and service level agreements (SLAs) are essential tools for formalizing risk controls. These documents should clearly outline expectations for medication quality, delivery timelines, data security, and incident reporting.
For pharmaceutical suppliers, contracts should address critical factors like temperature control during shipping, documentation of active pharmaceutical ingredient (API) sources, and immediate reporting of quality issues or recalls. For technology vendors, SLAs should specify system uptime requirements, data backup procedures, and disaster recovery protocols.
A report from the Office of the Director of National Intelligence highlights that supply chain risks extend beyond primary vendors to include subcontractors and suppliers at all levels [6]. This reinforces the importance of addressing the practices of all parties involved in the supply chain within contracts. By doing so, pharmacies can better safeguard their operations and ensure compliance throughout the vendor network.
Protecting Medication Safety and Supply Chain Security
Once a vendor risk management program is established, pharmacies need to focus on actionable steps that directly safeguard medication safety and maintain a secure, reliable supply chain. These practical measures bring risk management principles into everyday operations, ensuring patient care remains a top priority.
Medication Safety Controls
It’s essential to ensure vendors comply with current Good Manufacturing Practices (cGMP) and uphold high-quality standards throughout production and distribution. The U.S. operates under a "closed" drug distribution system, regulated by federal and state laws to protect the supply chain’s integrity [1]. The Drug Supply Chain Security Act (DSCSA) plays a key role here, requiring a robust electronic system to trace prescription drugs at the package level. Its purpose? To prevent harmful drugs from entering the supply chain, quickly identify any that do, and enable swift removal to protect patients [7].
Pharmacies must actively verify that vendors adhere to these requirements. This includes ensuring vendors provide electronic product tracing data, reporting illegitimate products to the FDA within 24 hours, and using serialization or anti-counterfeiting tools like physical-chemical identifiers in oral solid dosage forms. Additionally, the FDA’s Know Your Source initiative advises procuring medications exclusively from licensed sources to minimize risks [1]. These measures form the foundation for addressing other product-specific concerns, such as temperature-sensitive medications.
Managing Temperature-Sensitive Medications
Temperature-sensitive medications require meticulous handling to maintain their efficacy. Pharmacies must collaborate closely with vendors to define acceptable temperature ranges and establish clear procedures for monitoring and reporting any deviations. Adhering to manufacturer guidelines for storage and transport is non-negotiable when it comes to protecting medication quality.
Aligning Vendor Controls with Pharmacy Workflows
Incorporating vendor safety measures into daily pharmacy operations is crucial for maintaining supply chain security. By aligning DSCSA requirements with routine workflows, pharmacies can ensure seamless, compliant operations. This includes integrating package-level drug tracing and electronic data exchange with vendors and regulatory bodies [7]. Tools like the DSCSA portal simplify communication with the FDA and partners, streamlining the process.
Training pharmacy staff is equally important. Employees should be well-versed in DSCSA protocols, including how to identify illegitimate products, use the DSCSA portal, and report issues within the required 24-hour window [7]. Proper training ensures that everyone is prepared to handle potential risks effectively.
For additional guidance, pharmacies can turn to the APEC Supply Chain Security Toolkit for Medical Products. Developed with FDA support and updated regularly since 2017, this resource outlines best practices in areas like clinical pharmacy operations, good distribution practices, product security, and track-and-trace systems [8]. By using this toolkit, pharmacies can align vendor requirements with internal processes, enhancing formulary management, inventory control, and medication dispensing. As the FDA emphasizes, "protecting the integrity of the medical product supply chain is complex and requires a global, multi-layered approach that includes prevention, detection, and response strategies" [8].
sbb-itb-535baee
Using Censinet for Pharmacy Vendor Risk Management
Overseeing pharmacy vendor risks - whether it's ensuring medication safety, securing the supply chain, or staying compliant with regulations - requires a system that can handle complexity without adding unnecessary headaches. That’s where Censinet RiskOps™ steps in, offering healthcare organizations a streamlined, centralized platform tailored to the unique challenges of vendor management.
Simplifying Risk Assessment and Monitoring
Censinet makes evaluating and monitoring vendor risks more efficient throughout every stage of the vendor relationship. Powered by Censinet AI™, the platform speeds up risk assessments by allowing vendors to complete detailed questionnaires in just seconds. From there, the AI gets to work summarizing vendor evidence, integration details, and documentation. It also keeps an eye on fourth-party risks and generates concise risk summary reports. The result? Pharmacy risk teams can assess more vendors in far less time, all while maintaining high standards for medication safety and compliance.
What makes the system even more effective is its “human-in-the-loop” design. Automation handles the heavy lifting, but pharmacy risk teams remain in control with customizable rules and review processes. This balance allows teams to scale their operations without compromising the critical oversight needed to protect patient safety.
Automated Workflows for Seamless Collaboration
Censinet AI™ also ensures that risk assessment findings and tasks get to the right people quickly. For example, if a vendor assessment flags an issue with medication quality or supply chain security, the platform immediately notifies the relevant pharmacy staff, quality assurance teams, or compliance officers.
This automated workflow keeps everyone on the same page, ensuring risks are addressed promptly and efficiently. By coordinating communication across teams, the platform fosters continuous oversight and accountability, helping pharmacies maintain a strong, proactive stance in protecting patient care.
Conclusion
Main Takeaways from This Guide
This guide has explored essential strategies for managing pharmacy vendor risks, covering everything from vendor lifecycle management to integrating quality principles. At its core, effective vendor risk management safeguards patient safety while ensuring smooth pharmacy operations. Real-world examples, like a recent data breach that compromised the information of over 271,000 patients, highlight just how far-reaching the consequences of vendor failures can be [9].
The best results come from combining structured vendor management processes with ongoing monitoring and well-defined contractual controls. Borrowing quality risk management principles from pharmaceutical manufacturing offers a reliable way to identify, evaluate, and address vendor-related risks throughout the partnership. This approach is particularly important for handling challenges like temperature-sensitive medications, supply chain disruptions, and cybersecurity threats, ensuring these risks align with the day-to-day demands of pharmacy workflows.
While vendor oversight is undeniably critical, there’s a glaring gap: nearly 80% of organizations have formal vendor risk assessment programs, but about 30% lack dedicated staff to execute these processes [9]. This shortfall explains why many pharmacies are turning to technology-driven solutions, which allow them to scale their vendor risk management efforts without overburdening their teams.
How Technology Improves Risk Management
Technology has become an indispensable tool for addressing these challenges. By replacing outdated, paper-based methods with proactive, scalable systems, technology empowers pharmacy teams to stay ahead of potential risks. Specialized healthcare platforms enable comprehensive assessments, real-time vendor performance monitoring, and swift responses to new threats. For example, Cleveland Clinic has adopted AI and automation to strengthen its healthcare supply chain [4][10], showcasing how technology can enhance both efficiency and resilience.
The numbers tell a striking story: downstream entities affected by multi-party incidents outnumber the primary victims by over 800% [9]. This means a single vendor breach can ripple out to impact hundreds of healthcare organizations and hundreds of thousands of patients. As Stephen Stalker from McKesson aptly puts it:
"Maintaining a resilient supply chain is vital to serving providers and protecting patient access to life-saving medication" [4].
Technology makes this level of resilience achievable. With tools for continuous monitoring, automated workflows, and centralized documentation, pharmacy teams are better equipped to protect both medication safety and supply chain security. By blending strong risk controls with advanced tech solutions, pharmacies can ensure safer medication practices while building a more secure and reliable supply chain.
FAQs
What steps can pharmacies take to ensure medication quality and supply chain security?
Pharmacies can protect the quality of medications and strengthen their supply chain by conducting thorough risk evaluations for every vendor. This means checking vendor credentials, confirming adherence to FDA, DEA, and HIPAA regulations, and performing regular audits to keep track of their performance.
It's also wise for pharmacies to work with multiple suppliers to avoid over-reliance on a single source. Building strong, open partnerships with vendors and maintaining ongoing oversight can help spot risks early and ensure medications remain safe and reliable.
What cybersecurity risks do pharmacies face from their vendors?
Pharmacies are increasingly vulnerable to cybersecurity threats stemming from their vendors, which can have serious consequences for patient safety and disrupt daily operations. Common risks include ransomware attacks, data breaches, and software vulnerabilities that could expose sensitive patient and business information. On top of that, flaws in cloud-based systems or unauthorized access can threaten the integrity of medications and compromise the security of the supply chain.
To safeguard against these threats, pharmacies need to ensure their vendors adhere to strict security protocols and routinely evaluate their systems for potential weaknesses. Regular assessments and proactive measures can make a significant difference in maintaining both safety and operational continuity.
How does technology help improve pharmacy vendor risk management?
Technology significantly enhances pharmacy vendor risk management by streamlining complex tasks like risk assessments and due diligence. Automation not only cuts down on manual work but also ensures vendor evaluations are handled with greater consistency and accuracy.
On top of that, advanced tools support continuous monitoring of vendors. This means pharmacies can keep a close eye on compliance, security protocols, and overall performance in real time. By doing so, they can quickly spot and address potential issues, helping to maintain medication safety and protect the integrity of the supply chain.


