State Healthcare Licensing and Vendor Risk: Regulatory Compliance Across Jurisdictions
Post Summary
Navigating healthcare compliance across states is challenging. Each state has unique licensing rules, creating complexities for organizations and vendors working in multiple jurisdictions. Federal laws like HIPAA set minimum requirements, but state-specific regulations often add stricter obligations for licensing, data protection, and breach notifications. This makes vendor risk management essential, especially for those handling sensitive patient data across state lines.
Key takeaways:
- State licensing rules vary widely, requiring vendors to meet unique standards in every jurisdiction they operate.
- Federal rules like HIPAA and HITECH provide a baseline, but compliance with state-specific laws is equally critical.
- Vendor risk management frameworks should address state-specific licensing, privacy, and cybersecurity requirements.
- Technology solutions, like Censinet RiskOps, simplify compliance tracking and automate vendor assessments.
This guide covers how to build a vendor risk framework, conduct assessments, and integrate state-specific licensing into contracts and incident response plans. By centralizing oversight and leveraging technology, healthcare organizations can reduce compliance gaps and maintain regulatory alignment across states.
Healthcare Vendor Risk and Compliance Statistics 2024-2025
How State Licensing and Regulatory Differences Affect Vendor Risk
Navigating the regulatory world for healthcare vendors can feel like walking a tightrope. Federal laws provide a baseline, but state-specific requirements add layers of complexity that healthcare organizations must address. These overlapping regulations can create potential compliance gaps, especially for organizations operating in multiple states. Understanding the nuances of these federal and state rules is key to managing vendor risks effectively and crafting strategies that address both levels of governance. Let’s break down how these regulations intersect to shape vendor risk.
Federal Regulations and Their Limitations
Federal laws like HIPAA, HITECH, and CMS lay the groundwork for protecting patient data and maintaining security standards [4][5][7]. These regulations extend to vendors through requirements such as Business Associate Agreements (BAAs), which outline how vendors must handle sensitive health information. Other federal rules, including the Social Security Act (Sections 1128 and 1156 for OIG exclusions), the False Claims Act, and Stark Law, aim to prevent fraud and uphold integrity across healthcare programs [4][5][7][8].
However, while federal laws establish minimum standards, they leave room for states to impose stricter or more detailed requirements [2][7]. This creates a significant challenge: compliance with federal rules doesn’t automatically mean compliance at the state level. For example, HIPAA requires organizations to notify affected parties in the event of a data breach, but states like California have their own notification laws with stricter timelines and additional obligations [7]. These variations mean healthcare organizations must stay vigilant about state-specific rules, particularly when it comes to licensing.
State Licensing Rules and Vendor Compliance
State boards oversee licensing for certain healthcare services - like telehealth, pharmacy operations, and laboratory services - and their requirements can vary widely across jurisdictions [2]. Vendors supporting these services must comply with the licensing rules of each state where they operate. A telehealth vendor, for instance, must meet the unique licensing standards of every state in which it provides services, and these standards often differ significantly in scope and detail.
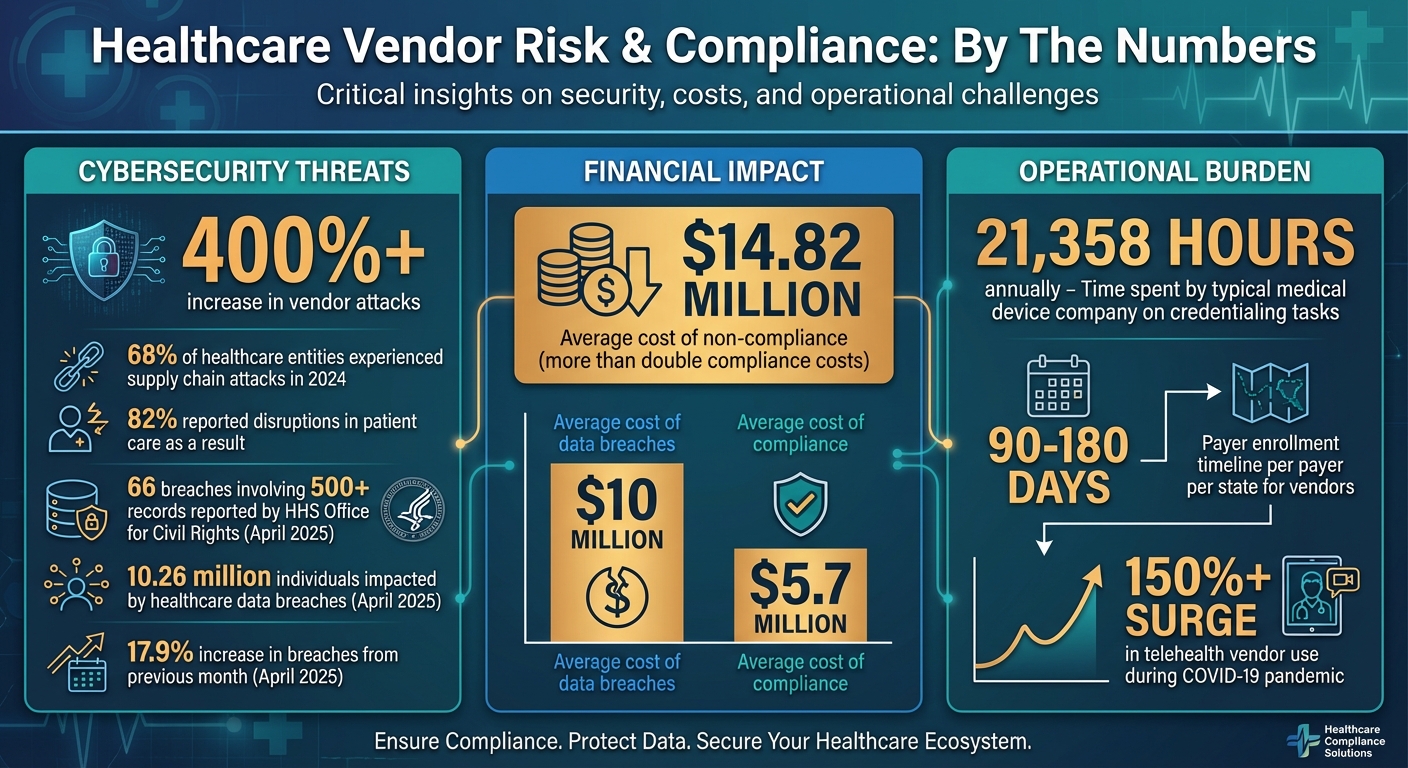
Credentialing adds another layer of complexity. Not only do credentialing requirements vary across states, but they can also differ between facilities within the same healthcare system [3]. For example, a typical medical device company spends a staggering 21,358 hours annually handling credentialing tasks across administrative, HR, and sales departments [3]. Beyond federal exclusion lists like the OIG's List of Excluded Individuals and Entities (LEIE), healthcare organizations are also responsible for monitoring vendors against state-specific exclusion lists. Failing to do so can lead to costly fines, as regulators increasingly crack down on organizations that work with excluded vendors [4].
State Privacy, Data Protection, and Cybersecurity Laws
State-level privacy and cybersecurity laws introduce yet another set of challenges for managing vendor risk. Many states have enacted their own data protection laws that go beyond federal requirements, adding more obligations for vendors. For instance, California's Consumer Privacy Act (CCPA) is one of the most comprehensive state privacy laws, requiring healthcare organizations and their vendors to navigate additional layers of compliance [7]. These laws often cover specific types of data - like biometric or genetic information - that federal regulations don’t fully address [6][7][8].
State laws also govern critical areas such as data breach notifications, public health reporting, and medical record retention, all of which vary by jurisdiction [7]. The stakes are high: healthcare data breaches in April 2025 alone saw a 17.9% increase from the previous month, impacting 10.26 million individuals. The HHS' Office for Civil Rights reported 66 breaches involving 500 or more records during that time [7]. Compounding the issue, 68% of healthcare entities experienced supply chain attacks in 2024, and 82% reported disruptions in patient care as a result [2]. These statistics underscore the importance of understanding state-specific cybersecurity requirements to protect patient data and maintain smooth operations.
The complexities of these state-level regulations play a critical role in shaping vendor risk management frameworks, which we’ll explore in the next section.
Building a Vendor Risk Framework for Multiple States
Managing vendor risks across multiple states comes with its own set of challenges. The key is to establish a framework that balances consistency with flexibility. You need standardized processes that work universally, but they must also accommodate the unique regulations and requirements of each state. Without this balance, healthcare organizations could face compliance gaps, redundant efforts, or inconsistent oversight of vendors. A well-structured framework provides clear governance roles, unified policies that adapt to state-specific needs, and technology solutions to keep everything organized. This approach ensures effective oversight and reduces the risk of non-compliance.
Assigning Roles for Vendor Risk Governance
Defining roles clearly is essential for accountability and smooth operation. Different teams within your organization play distinct roles in managing vendor risks:
- Compliance teams handle state-specific licensing requirements and stay updated on regulatory changes.
- Legal teams review vendor contracts to ensure they include the correct jurisdictional clauses.
- Security teams focus on assessing cybersecurity risks and monitoring vendor access to sensitive data.
- Procurement and operations teams oversee vendor relationships and escalate state-specific concerns as needed.
This structure works best when centralized oversight is paired with localized expertise. For instance, a compliance officer in California should be well-versed in CCPA-related vendor obligations, while a central governance committee makes the final decisions on vendor approvals. This collaboration ensures that jurisdictional issues are identified quickly and resolved efficiently, with input from both local and organization-wide perspectives.
Creating a Central Policy Framework with State-Specific Additions
A unified policy framework serves as the foundation of vendor risk management. Start with standardized credentialing policies that outline vendor approval processes, required documentation, background checks, training certifications, and compliance with federal regulations like HIPAA. These baseline policies apply to all vendors, regardless of their location.
From there, layer in state-specific requirements to address geographic nuances. For example, you might:
- Tag documents by jurisdiction to streamline tracking.
- Build approval workflows that include regional reviewers familiar with local regulations.
- Set risk thresholds that account for the complexity of state-specific rules.
Precise vendor classification is critical when dealing with state-specific obligations. In addition to using traditional risk categories like "high", "medium", and "low", consider adding criteria like "geographic regulatory scope." This helps identify vendors that operate in multiple states and may face additional compliance challenges. For instance, a telehealth vendor working across several states would require extra scrutiny to ensure compliance with licensing requirements in each jurisdiction.
Using Technology to Track Vendors and Risks
Technology plays a crucial role in managing vendor data and compliance. Platforms like Censinet RiskOps centralize vendor records, agreements, policies, and compliance documentation, making everything easily accessible. These tools automate compliance workflows, organize vendors by jurisdiction, and provide continuous monitoring to ensure adherence to federal regulations like HIPAA and HITECH, as well as state-specific laws such as CCPA and breach notification requirements.
Conducting Vendor Assessments for Licensing and Compliance
Vendor assessments put your compliance framework into action by ensuring adherence to federal and state licensing standards. The primary goal? Spot risks before they escalate into compliance violations. These assessments focus on critical areas like data security, licensing credentials, and operational practices. A solid approach combines standardized questionnaires with tailored reviews of state-specific requirements, leaving no room for oversight. Here’s a look at effective risk-based assessment practices.
Risk-Based Vendor Assessment Methods
Start by categorizing vendors based on their risk level and geographic reach. High-risk vendors - those dealing with protected health information (PHI), offering clinical services, or operating in multiple states - demand deeper scrutiny. Federal standards like the CMS Conditions of Participation (CoPs) set the baseline, ensuring vendors meet requirements for patient safety, infection control, and data security to maintain Medicare/Medicaid funding [1]. Beyond these federal rules, incorporate state-specific licensing checks, especially in states like California, Texas, and Florida, which have detailed credentialing regulations [1].
Use a mix of questionnaires and onsite audits to verify compliance [1]. Questionnaires should cover essentials like licensing status, background checks, and training certifications. For vendors operating across state lines, include questions about their understanding of state-specific data protection laws. Onsite audits are particularly useful for high-risk vendors, allowing you to verify physical security measures, observe data handling practices, and confirm that their operations align with documented policies.
What to Assess for Licensing and Compliance
Focus on key areas during your assessments. Start with identity verification and background checks - some states even require fingerprinting [10]. Cybersecurity controls should meet standards for encryption, access management, and incident response. When it comes to PHI handling, ensure practices align with HIPAA and state privacy laws like the CCPA. Additionally, evaluate business continuity plans to confirm vendors can continue operations during disruptions without jeopardizing patient care or data security [10].
Vendor compliance isn’t a one-and-done task - it requires ongoing monitoring [4]. Keep track of license expiration dates and regulatory updates that might impact compliance. For telehealth vendors, whose use surged by over 150% during the COVID-19 pandemic [9], confirm that all providers hold valid licenses in every state where they deliver care. Remember, payer enrollment for vendors can take 90–180 days per payer per state [11], so build these timelines into your onboarding schedule to avoid revenue delays.
Automating Assessments with Censinet RiskOps

Manually managing assessments across multiple states can be overwhelming. Censinet RiskOps simplifies the process by automating tasks like distributing questionnaires, collecting evidence, and generating risk reports. The platform centralizes vendor documentation, tracks compliance in real time, and flags missing credentials or inconsistencies before they become issues. Automated workflows ensure findings are routed to the right teams - whether compliance, legal, or security - based on jurisdictional requirements, giving state-specific concerns the attention they need.
Censinet AI takes automation a step further. Vendors can complete security questionnaires in seconds, while the platform summarizes evidence, documentation, and key product details, including fourth-party risks. Risk teams remain in control with customizable rules and structured reviews, ensuring automation supports critical decision-making rather than replacing it. This approach significantly reduces the time spent on vendor assessments while maintaining the rigor required to meet both federal and state compliance standards. It’s a practical solution that strengthens your multi-state compliance strategy without sacrificing thoroughness.
sbb-itb-535baee
Adding State Licensing Requirements to Vendor Contracts and Incident Response
To strengthen your vendor risk framework, it's essential to integrate state licensing requirements into contracts and establish solid incident response protocols. After assessing vendors, the next step is securing compliance through well-crafted agreements and preparing for multi-state incident management. Vendor contracts must go beyond federal mandates like HIPAA, the Stark Law, and the Anti-Kickback Statute by addressing state-specific licensing and regulatory needs [12][13][14]. Here's how to incorporate these elements into contracts and create effective response plans that span multiple jurisdictions.
Contract Clauses for Licensing and Compliance
Contracts play a key role in holding vendors accountable to state and federal standards. Start by including licensing warranties that require vendors to maintain all necessary state licenses and certifications throughout the contract term. Vendors should also confirm compliance with state and federal exclusion lists, as your organization bears the ultimate responsibility for monitoring their status [4]. For vendors handling sensitive data like Protected Health Information (PHI) or Personally Identifiable Information (PII), Business Associate Agreements (BAAs) must include clauses that address both HIPAA and relevant state privacy laws [12][14].
Clearly define notification timelines for vendors to report licensing changes, compliance issues, or security incidents. These timelines should align with the strictest state requirements in your operational footprint, as reporting deadlines can vary widely across jurisdictions. Additionally, require vendors to provide documentation of disaster recovery and incident response plans, particularly if they manage high-risk data like PHI or PII [7][4]. Contracts should explicitly state that vendors must comply with all relevant state laws - not just federal ones - and outline penalties for non-compliance, such as financial repercussions or contract termination.
Planning Incident Response Across Multiple States
A proactive approach to incident response is critical, especially when managing compliance across multiple states. Each state has unique reporting rules for data breaches, infectious diseases, and public health emergencies, which add layers of complexity to federal HIPAA requirements [7][2]. For example, in April 2025, healthcare data breaches rose by 17.9% compared to the previous month, impacting over 10.26 million individuals. The HHS Office for Civil Rights recorded 66 breaches involving 500 or more records during that time [7].
To address these challenges, develop an incident response plan that includes patient notification protocols tailored to both state-specific laws and federal HIPAA regulations [7]. Establish procedures for reporting incidents to state and local agencies, ensuring compliance with their unique formats, deadlines, and documentation standards. Implement a centralized incident management system to log, track, and document all incidents and compliance issues. This system should categorize violations by severity and route them to the appropriate team - for instance, escalating a Level 3 HIPAA violation directly to your compliance department [15]. This tiered approach ensures a swift, organized response while meeting both state and federal requirements.
Maintaining Audit Trails with Censinet RiskOps
Keeping thorough audit trails is essential for demonstrating compliance during regulatory reviews. Non-compliance costs an average of $14.82 million - more than double the $5.7 million average cost of compliance [2]. Tools like Censinet RiskOps simplify this process by maintaining detailed records of compliance activities, vendor assessments, contract reviews, and incident responses [16][17]. Every action, from distributing questionnaires to tracking license renewals and logging incidents, is automatically documented.
This centralized system is especially valuable when proving compliance across multiple states. Censinet RiskOps ensures you have a complete record of your due diligence, making it easier to respond to auditor requests or regulatory inquiries. Its structured workflows and consistent documentation practices transform your compliance program into a dynamic, evolving system that adapts to new laws and circumstances, rather than relying on outdated, static policies [4]. With all records stored in one place, your organization can confidently demonstrate its efforts to manage vendor risks and maintain compliance across every jurisdiction in which it operates.
Conclusion
Navigating vendor risks and regulatory compliance across multiple states is no small feat, especially as healthcare organizations grapple with rising cyber threats and increasingly strict regulations. Consider this: vendor attacks have skyrocketed by over 400%, with 68% of organizations reporting supply chain attacks in 2024. Meanwhile, the financial toll of data breaches and non-compliance has soared to an average of nearly $10 million and $14.82 million, respectively[5][2]. These numbers underscore the pressing need for a smarter, more unified approach to risk management.
The foundation of success lies in standardizing vendor risk evaluations while allowing flexibility to address unique state licensing and privacy laws. Fragmented compliance efforts - where teams operate in silos - lead to inefficiencies, duplicated work, and misaligned strategies[18]. Instead, adopting an enterprise risk management framework brings everything under one roof. This approach ensures consistency, fosters transparency, and provides real-time insights into vendor compliance across the board.
As regulations shift and evolve, continuous monitoring becomes non-negotiable. Without it, healthcare facilities risk fines, audits, and even threats to patient safety due to inconsistent documentation and outdated processes[2][10]. Centralizing information sharing is another critical piece of the puzzle, enabling teams to stay ahead of regulatory updates, address vendor concerns, and tackle security issues before they spiral out of control.
Enter Censinet RiskOps - a solution designed to simplify these challenges. By automating compliance workflows and centralizing audit trails, Censinet RiskOps allows organizations to stay ahead of shifting regulations. Instead of relying on rigid, outdated policies, this platform supports a dynamic compliance program that adapts to new laws and operational needs. It’s a game-changer for managing multi-state operations with confidence.
Ultimately, a well-structured vendor risk framework does more than just ensure compliance - it safeguards patient care, keeps operations running smoothly, and demonstrates a clear commitment to regulatory standards. With the right tools and strategies in place, healthcare organizations can transform the complexities of multi-jurisdictional compliance into a strategic advantage, turning challenges into opportunities. By combining standardized evaluations with state-specific adaptability, the path to compliance becomes not just manageable but a source of strength.
FAQs
What are the best practices for managing vendor compliance in healthcare across multiple states?
Managing vendor compliance across multiple states can be a challenging task for healthcare organizations, but implementing a centralized system can make a world of difference. Such a system allows you to track credentials, monitor license expirations, and stay on top of state-specific requirements - all in one place. Adding automation to the mix can lighten the administrative load and help reduce the chances of errors slipping through.
Clear communication is another key piece of the puzzle. When vendors have a solid understanding of regulatory expectations through well-defined policies, compliance becomes much smoother. Regular audits and ongoing staff training also play a crucial role in keeping everything on track. And let’s not forget the power of technology - it can simplify processes and make compliance efforts more efficient. By staying organized and taking a proactive approach, healthcare organizations can handle the complexities of multi-state regulations with greater ease and confidence.
How do federal and state healthcare regulations differ?
Federal healthcare regulations set nationwide standards in key areas like patient privacy (think HIPAA), fraud prevention (such as the False Claims Act and Stark Law), and anti-kickback rules. These regulations create a unified framework to protect patient rights and ensure accountability across the healthcare industry.
State regulations, however, differ depending on the jurisdiction. They often focus on local licensing requirements, background checks, and specific compliance rules for healthcare providers and vendors. While federal laws provide the broad guidelines, states add their own rules to address the unique needs of their healthcare systems, including operational and licensing requirements for healthcare organizations.
How does Censinet RiskOps help healthcare organizations track compliance automatically?
Censinet RiskOps makes compliance tracking easier by automating the monitoring of vendor documents, certifications, and regulatory requirements in real time. It flags potential issues like expired certifications, discrepancies, or non-compliance, helping your organization stay aligned with evolving regulations.
By cutting down on manual work and reducing the risk of errors, this tool ensures healthcare organizations can maintain regulatory consistency, even across different regions.


