Surgical Equipment Vendor Risk Management: Operating Room Safety and Reliability
Post Summary
Operating rooms depend on reliable surgical equipment to ensure patient safety and procedure success. Faulty devices, cybersecurity risks, and supply chain disruptions can lead to severe complications, delays, and increased costs. Many healthcare facilities struggle to thoroughly assess vendor risks, leaving critical vulnerabilities unaddressed.
Key risks include:
- Clinical Safety: Equipment malfunctions can cause injuries, burns, fires, or other complications during surgery.
- Cybersecurity: Connected devices are prone to breaches, exposing patient data or causing operational failures.
- Supply Chain: Relying on sole-source vendors or poorly managed suppliers can disrupt surgical operations.
To mitigate these risks, healthcare organizations must implement structured vendor risk management programs. This includes evaluating supplier reliability, product quality, regulatory compliance, and cybersecurity measures. Regulatory frameworks like FDA's Quality Management System Regulation (QMSR) and ISO standards provide clear guidelines for managing these risks.
Platforms like Censinet simplify vendor assessments by automating processes, consolidating data, and providing real-time oversight. By addressing vendor risks systematically, healthcare providers can protect patients, reduce disruptions, and maintain operational efficiency.
Types of Surgical Equipment Vendor Risks
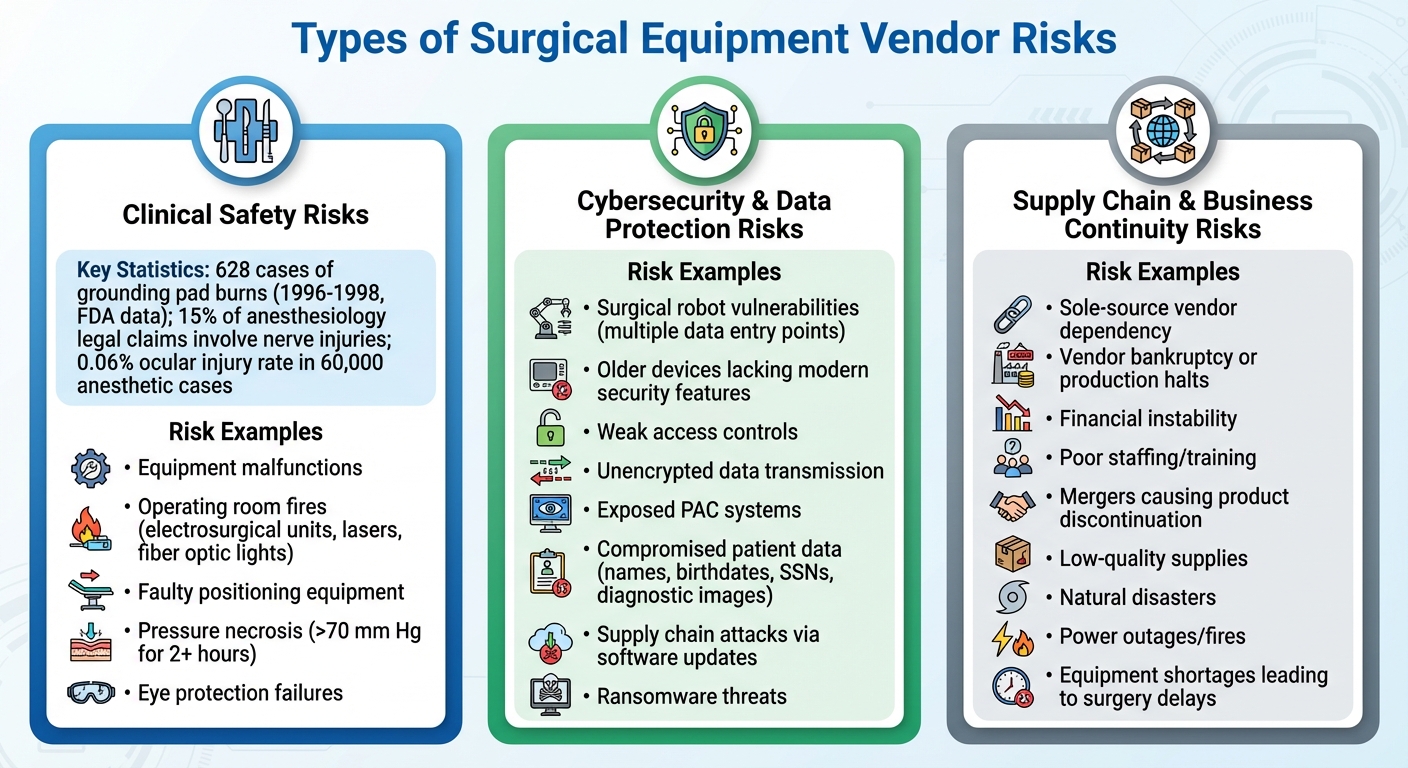
Three Main Categories of Surgical Equipment Vendor Risks in Healthcare
Surgical equipment vendors play a critical role in ensuring operating room efficiency and patient safety. However, they also introduce specific risks that can jeopardize these outcomes. These risks fall into three main categories: clinical safety, cybersecurity, and supply chain vulnerabilities.
Clinical Safety Risks
Equipment malfunctions during surgery can pose immediate and severe dangers. For example, between 1996 and 1998, the FDA recorded 628 cases of grounding pad burns caused by electrosurgical devices. These burns occurred due to issues like pads losing contact, being improperly placed over bony areas, or accidental electrode activation [2].
Operating room fires are another critical concern. Devices like electrosurgical units, lasers, and fiber optic lights can ignite surgical drapes or alcohol-based preparations in oxygen-enriched environments. According to the Anesthesia Patient Safety Foundation, nearly all such fires are preventable with proper precautions [3].
Faulty positioning equipment can also lead to nerve injuries, with 15% of anesthesiology-related legal claims involving ulnar, radial, or brachial plexus damage [2]. Insufficient padding can cause pressure necrosis when tissue is exposed to pressure exceeding 70 mm Hg for over two hours [2]. Eye protection failures have been linked to ocular injuries in 0.06% of 60,000 anesthetic cases [2]. These examples highlight the critical need for reliable equipment to ensure patient safety during surgical procedures.
Cybersecurity and Data Protection Risks
As surgical equipment becomes more connected, cybersecurity threats have emerged as a significant concern. Devices like surgical robots integrate multiple data sources, hardware, and software, creating numerous entry points for cyberattacks [4]. A breach could have devastating consequences, including physical harm during procedures [4].
Older medical devices often lack modern security features, while newer ones may come with weak access controls or unencrypted data transmission [5]. Medical imaging systems used for surgical planning are particularly vulnerable, with some Picture Archiving and Communication (PAC) systems remaining completely exposed. This leaves sensitive patient data - such as names, birthdates, Social Security numbers, and diagnostic images - accessible to attackers [5]. Altered radiology images, for instance, could mislead surgeons and compromise patient outcomes [5].
Software updates and patches are another potential weak spot, opening the door to supply chain attacks or man-in-the-middle compromises [4]. Cloud-based features, such as video recording, performance tracking, and mobile access, further expand the risk. With health information fetching high prices on the black market, ransomware groups have adopted aggressive tactics like encrypting data, threatening to publish it, and targeting affected patients [5]. These vulnerabilities not only endanger patient data but also the safe operation of critical surgical equipment.
Supply Chain and Business Continuity Risks
Disruptions in the supply chain can have far-reaching consequences for surgical operations. Relying on a single supplier for crucial components is particularly risky. If that supplier faces bankruptcy, halts production, or encounters natural disasters, finding an alternative vendor can be a lengthy process, delaying surgeries and disrupting operations [7].
Vendor instability adds another layer of risk. Financial troubles, poor staffing, inadequate training, or flawed processes within a vendor's operations can lead to delays in equipment delivery or maintenance [6]. Strategic shifts, such as mergers or management changes, might result in the discontinuation of essential products [6]. Low-quality supplies from vendors can directly compromise patient safety during surgeries [7]. Additionally, unexpected events like power outages, fires, or financial crises can prevent vendors from meeting their obligations, leading to equipment shortages and postponed surgeries [6]. These disruptions can severely impact patient care and operating room efficiency.
Regulations and Standards for Surgical Equipment Risk Management
Healthcare organizations must navigate strict regulatory frameworks when managing risks tied to surgical equipment vendors. In the United States, the U.S. Food and Drug Administration (FDA) enforces these regulations under the Federal Food, Drug, and Cosmetic Act. These rules are outlined in Title 21 of the Code of Federal Regulations (CFR) Parts 800–1299 [9]. These guidelines shape how hospitals and surgical centers procure and oversee equipment from vendors, forming the foundation for surgical vendor risk management practices.
FDA Quality Management System Regulation

The FDA's Quality Management System Regulation (QMSR), detailed in 21 CFR 820.10(a), requires medical device manufacturers to implement a quality management system that aligns with ISO 13485:2016, a standard formally recognized by the FDA [10]. This isn't just a recommendation - it’s a regulatory mandate for surgical equipment manufacturers operating within the U.S. [10]. The QMSR emphasizes risk management across the device lifecycle, including supplier evaluation. According to ISO 13485:2016 Clause 7.4 (Purchasing), manufacturers must evaluate and select suppliers based on the level of risk associated with the medical device [10]. This ensures that suppliers are carefully vetted before their equipment reaches operating rooms.
Risk Management Standards
Effective risk management involves identifying, assessing, controlling, and reviewing risks associated with medical devices, as outlined in ISO 14971:2019, which was reaffirmed in 2025 [8]. This standard complements the risk management principles embedded in both ISO 13485 and the FDA's QMSR.
Healthcare organizations should scrutinize how vendors comply with the FDA's QMSR, focusing on supplier evaluation processes, purchasing controls, and risk management for purchased products [10]. The FDA's 2024 Medical Devices Quality System Regulation Amendments (FR 89‑7496) further emphasize the integration of risk management within ISO 13485, bringing greater clarity to these requirements [10].
These regulatory frameworks allow manufacturers to adapt risk management protocols to suit the specific types of surgical equipment being produced.
Applying Standards to Surgical Equipment
Surgical equipment is subject to different regulatory requirements depending on its risk profile. For example, devices reliant on software - like surgical robots or networked imaging systems - must adhere to ISO 13485:2016 Clause 7.5, which mandates that software validation and revalidation be proportional to the risks associated with its use [10]. Design inputs for such devices must incorporate risk management outputs, while design outputs must specify features critical for safe and effective use [10].
If nonconforming products are identified after delivery or use, manufacturers are required to take corrective actions that address potential risks to patient safety [10]. By thoroughly documenting and regularly reviewing risk management activities, healthcare organizations can ensure compliance and maintain the safety and functionality of surgical equipment throughout its lifecycle. These clear regulatory guidelines help align every aspect of equipment design and supply with stringent vendor risk management practices.
Building a Surgical Equipment Vendor Risk Management Program
Creating a vendor risk management program for surgical equipment involves establishing structured, repeatable processes to ensure patient safety and maintain smooth surgical operations. Moving away from informal evaluations, healthcare organizations should adopt formalized procedures that align with existing quality and safety standards. These efforts tie into the regulatory frameworks mentioned earlier, reinforcing the broader goal of surgical safety.
Setting Up Governance and Ownership
Start by developing a Risk Management Plan (RMP) that clearly defines the program's scope, outlines stakeholder responsibilities, and sets risk criteria and analysis methods[11]. This plan is not just a best practice; it’s a key requirement for meeting regulatory standards like ISO 14971[11]. To maintain consistent oversight, integrate the RMP with your Quality Management System. Assign specific responsibilities to key stakeholders to ensure every aspect of vendor risk is thoroughly managed.
Creating Standard Risk Assessment Processes
Establish standardized processes for risk assessment. Use clear risk criteria and schedule regular monitoring to allow for timely reassessments, ensuring risks are continuously evaluated and addressed.
Implementing Technical and Operational Controls
Introduce unified operational controls, such as a single vendor policy across all facilities. This approach simplifies updates, ensures consistent compliance, and prioritizes the safety of both patients and staff[1]. By standardizing these controls, healthcare organizations can better manage vendor-related risks while maintaining high operational standards.
sbb-itb-535baee
Using Censinet for Surgical Equipment Vendor Risk Management
In the healthcare world, managing vendor risks is essential to ensure the safety of operating rooms and the delivery of quality care. Censinet offers a single platform designed to simplify vendor risk management while adhering to strict healthcare safety and security standards. By building on established risk management practices, Censinet delivers tools that give healthcare organizations precise control over vendor assessments. Let’s take a closer look at how Censinet’s platform strengthens risk management for surgical equipment vendors.
Censinet RiskOps™ for Vendor Risk Management

Censinet RiskOps™ brings vendor risk assessments under one roof, streamlining the process from initial evaluations to ongoing monitoring and cybersecurity benchmarking. By using standardized criteria, it ensures vendors in the surgical equipment supply chain are thoroughly evaluated. This approach enables healthcare organizations to make informed, data-driven decisions while addressing risks related to clinical safety, cybersecurity, and supply chain reliability.
Censinet AI for Workflow Automation and Risk Governance
Censinet AI™ takes the tedious work out of vendor assessments by automating repetitive tasks. Vendors can complete security questionnaires in seconds, thanks to features that summarize documents, extract critical security details, and log compliance information. It generates detailed risk reports while giving organizations the flexibility to maintain human oversight through customizable rules and review workflows.
Unified Risk Dashboard for Complete Oversight
The unified risk dashboard offers a real-time view of vendor security and operational risks by consolidating data from standardized assessments. This centralized tool helps healthcare organizations quickly address emerging issues, allocate resources effectively, and make smarter vendor management decisions. With everything in one place, staying on top of vendor risks becomes much more manageable.
Conclusion
Ensuring the safety of surgical equipment vendors is essential for protecting patients and maintaining a secure operating room environment. When vendors fail to meet standards in areas like clinical safety, cybersecurity, or supply chain reliability, the impact can be felt across the entire healthcare system. As Shweta Dhole from TrustCloud emphasizes:
"A breach at one vendor can cascade through your supply chain; an outdated tool may become the weakest link in your cyber defenses" [12].
This highlights the importance of implementing a proactive and well-structured approach to managing vendor risks.
A comprehensive vendor risk management program equips healthcare organizations to pinpoint vulnerabilities before they escalate into significant issues. This involves keeping a close eye on software updates, access controls, and conducting regular security audits of vendor systems. Without these measures, organizations risk financial setbacks, legal challenges, and a loss of both reputation and patient trust. By addressing these areas systematically, healthcare providers can mitigate risks tied to clinical safety, cybersecurity, and supply chain operations.
Technology plays a key role in scaling these efforts. For instance, platforms like Censinet streamline vendor assessments, providing real-time insights into potential risks. By combining standardized workflows, automation, and human oversight, healthcare teams can manage risks more effectively and efficiently.
FAQs
What steps can healthcare organizations take to assess and manage risks from surgical equipment vendors?
Healthcare organizations can better handle risks tied to surgical equipment vendors by putting a comprehensive risk assessment process in place. Start by pinpointing major risk areas - think financial stability, compliance concerns, operational challenges, and reputational impacts. Develop clear criteria to evaluate vendors’ products, services, and overall performance. Then, categorize vendors by their risk levels so you can focus efforts on the ones that pose the greatest potential issues.
It's also crucial to keep risk management plans up to date as circumstances evolve. Tools like Failure Modes Effect Analysis (FMEA) and supplier scorecards can help assess and address risks more effectively. By consistently monitoring vendor compliance and performance, healthcare organizations can maintain reliability, ensure safety, and strengthen their supply chains - all of which are vital for protecting both patients and day-to-day operations.
What cybersecurity risks are associated with connected surgical devices?
Connected surgical devices are not immune to cybersecurity threats. They can be targets for unauthorized access, data breaches, and even device tampering. These risks aren't just technical - they can directly impact patient safety and disrupt the smooth operation of procedures in the operating room.
To address these challenges, it's crucial to implement strong security measures and conduct regular evaluations. These steps help maintain the integrity and reliability of surgical equipment, especially in high-stakes environments like critical care settings.
What are the benefits of using a single vendor for surgical equipment risk management?
Using one vendor for surgical equipment offers several advantages. It ensures consistent quality, making it easier to maintain high standards across all procedures. It also simplifies oversight and makes accountability clearer, which can be crucial in high-stakes environments like the operating room.
Partnering with a single reliable vendor helps healthcare facilities streamline their processes and improve communication. This approach not only reduces variability but also allows potential risks to be managed more effectively, contributing to safer operations and better patient care.


