Ultimate Guide to GDPR Audits in Healthcare
Post Summary
A GDPR audit in healthcare is a comprehensive review of how healthcare organizations collect, process, and protect patient data to ensure compliance with GDPR regulations.
GDPR audits help protect sensitive patient data, ensure compliance with EU regulations, avoid fines, and build trust with patients and stakeholders.
Key steps include data mapping, reviewing consent practices, ensuring robust security measures, and maintaining thorough documentation.
Challenges include managing sensitive health data, navigating complex regulations, ensuring vendor compliance, and maintaining transparency with patients.
Penalties can include fines up to €20 million or 4% of global annual turnover, reputational damage, and loss of patient trust.
Sustaining compliance requires regular audits, staff training, updated documentation, and embedding privacy into organizational culture.
GDPR compliance is mandatory for any healthcare organization handling EU patient data, even if based outside Europe. This regulation enforces strict safeguards for sensitive health data, with penalties reaching up to €20 million or 4% of global revenue. Key requirements include:
- Data Protection Impact Assessments (DPIAs): Mandatory for high-risk activities like large-scale patient data processing.
- Core GDPR Principles: Lawfulness, transparency, data minimization, accuracy, storage limitation, confidentiality, and accountability.
- Special Safeguards for Health Data: Encryption, role-based access, pseudonymization, and secure data handling.
- Patient Rights: Data access, erasure, portability, and objection.
- Breach Notifications: Required within 72 hours, far stricter than HIPAA’s 60-day rule.
This guide outlines how to prepare for GDPR audits by creating a data inventory, conducting risk assessments, updating policies, and implementing technical controls. Regular audits and tools like Censinet RiskOps™ can simplify compliance, ensuring healthcare organizations meet GDPR standards while safeguarding patient trust.
GDPR Requirements for Healthcare Organizations
GDPR vs HIPAA Compliance Requirements for Healthcare Organizations
The General Data Protection Regulation (GDPR) outlines seven key principles that healthcare organizations must follow when managing patient data: lawfulness, fairness, and transparency; purpose limitation; data minimization; accuracy; storage limitation; integrity and confidentiality; and accountability. These principles shape how electronic health records (EHRs), patient portals, and clinical documentation systems are designed and maintained. For example, transparency requires healthcare providers to give clear, plain-language explanations of how patient data is used and stored, while purpose limitation ensures that data isn't repurposed without proper legal justification.
Data minimization focuses on collecting only the information necessary for diagnosis and treatment, while accuracy emphasizes keeping patient records up to date and fixing errors promptly. Storage limitation means medical records should only be retained for as long as required by clinical or legal standards, with secure deletion or archiving afterward. To maintain integrity and confidentiality, organizations rely on safeguards like encryption, secure telehealth platforms, role-based access controls, and regular security audits. Finally, accountability involves documenting all data processing activities, policies, and conducting Data Protection Impact Assessments (DPIAs). These principles serve as the foundation for actionable steps in healthcare data management.
Core GDPR Principles in Healthcare
Transparency is put into practice by using layered privacy notices at key points, such as during registration, on patient portals, and in telehealth apps. These notices must clearly outline what data is collected, why it is needed, the legal basis for its use, how long it will be kept, and who to contact with privacy concerns. To meet data minimization requirements, EHR templates and clinical questionnaires should be routinely reviewed to remove unnecessary fields that don't directly aid in patient care. Storage limitation is enforced by implementing formal retention policies aligned with clinical and legal standards, as well as automated rules that archive or securely delete inactive records, including those in backups or test systems.
Accountability is achieved through maintaining a comprehensive Record of Processing Activities (RoPA), which details who processes patient data, for what purpose, and under which legal basis. This ensures healthcare organizations are always prepared for GDPR audits.
Understanding these principles is critical before addressing the additional safeguards required for handling sensitive health data.
Special Category Health Data Under GDPR
Health data falls under GDPR's "special category data", which includes information about a person's physical or mental health, such as diagnoses, treatments, lab results, genetic data, and biometric identifiers. This category also covers data stored in EHRs, transmitted via medical devices, shared through health information systems, or collected by wellness apps. Because of the sensitive nature of this data, healthcare organizations must implement strict measures like role-based access, multi-factor authentication, encryption, pseudonymization, secure network segmentation, and robust backup and disaster recovery plans.
For direct patient care, the legal basis for processing often includes public interest or contractual obligations. Administrative tasks like billing typically rely on contractual or legal obligations, while activities like clinical research or quality improvement may use public health or scientific research as their basis - provided safeguards like pseudonymization and ethics approvals are in place. For non-essential digital services, such as wellness newsletters, explicit patient consent is required. Additionally, a DPIA is mandatory for any large-scale or high-risk processing of special category data, and all legal bases must be clearly documented in the RoPA.
To better understand GDPR compliance in healthcare, it's helpful to explore how it compares to HIPAA.
GDPR and HIPAA: Differences and Overlaps
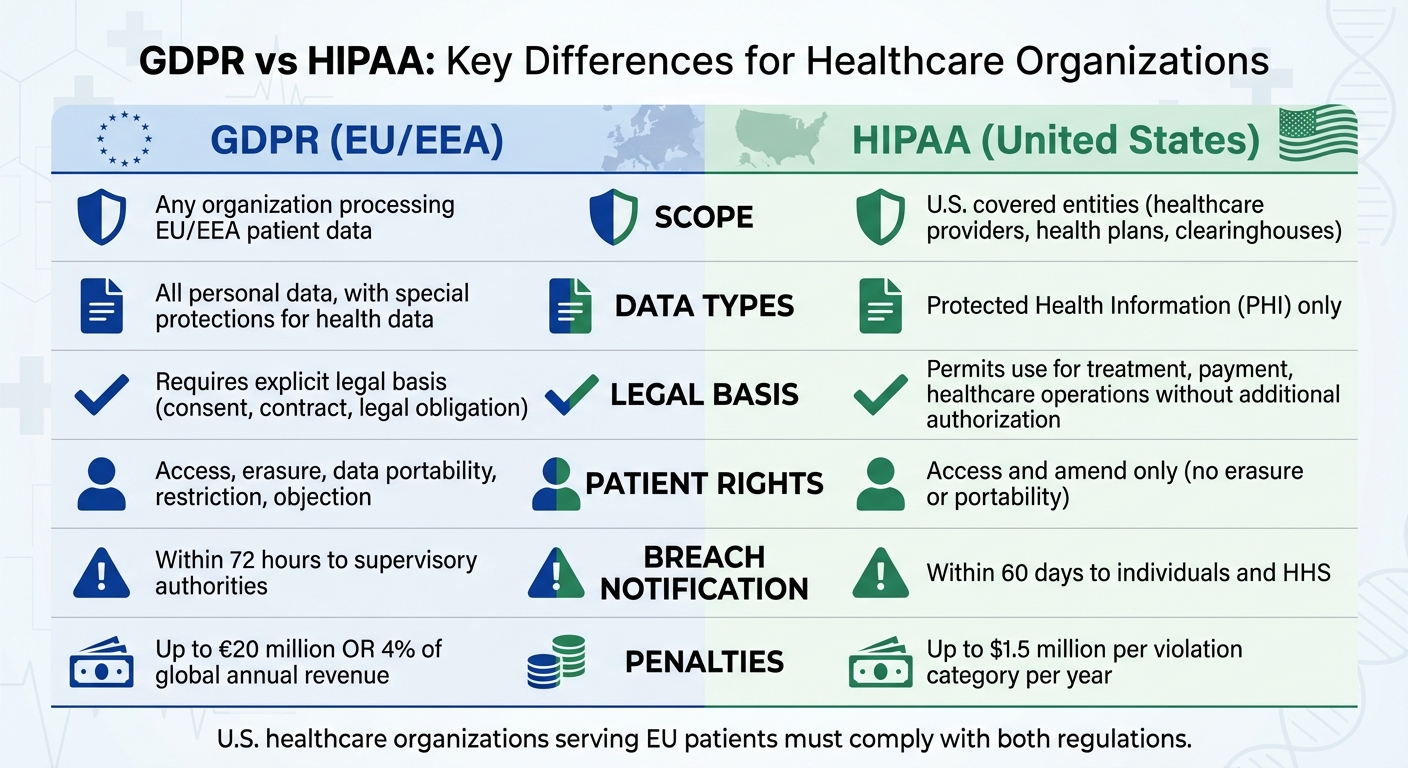
| Aspect | GDPR | HIPAA |
|---|---|---|
| Scope | Covers any organization processing EU/EEA patient data | Applies to U.S. covered entities (healthcare providers, health plans, clearinghouses) |
| Data Types | Covers all personal data, with special protections for health data | Focuses specifically on Protected Health Information (PHI) |
| Legal Bases | Requires an explicit legal basis for processing (e.g., consent, contract, legal obligation) | Permits use and disclosure for treatment, payment, and healthcare operations without additional patient authorization |
| Patient Rights | Grants rights such as erasure, data portability, restriction, and objection | Provides rights to access and amend, but does not include erasure or portability |
| Breach Notification | Requires notification to supervisory authorities within 72 hours | Requires notification within 60 days to affected individuals and the U.S. Department of Health and Human Services (HHS) |
| Penalties | Fines of up to €20 million or 4% of global annual revenue | Fines of up to $1.5 million per violation category per year |
For U.S. healthcare organizations that serve EU patients, compliance with both GDPR and HIPAA is essential. While HIPAA allows certain routine uses of Protected Health Information (PHI), GDPR demands a clear legal basis for all data processing activities and provides broader patient rights. GDPR also requires faster breach notifications and stricter data management practices.
Meeting GDPR's high standards not only strengthens data privacy but also supports overall regulatory compliance, including HIPAA adherence. Tools like Censinet RiskOps™ can simplify compliance by automating data mapping, maintaining a detailed RoPA, and ensuring all necessary safeguards are in place. This integrated approach helps protect patient data while easing the audit process.
How to Prepare for a GDPR Audit
Getting ready for a GDPR audit starts with assembling the right team and assigning clear responsibilities. Begin by appointing a Data Protection Officer (DPO) to lead your GDPR readiness efforts. This team should include representatives from IT, legal, clinical, and vendor management. An essential first step is determining whether your organization acts as a controller, processor, or both for each processing activity. Gather all relevant policies, contracts, and security documentation that can serve as evidence during the audit. For U.S. healthcare organizations, it’s also important to identify which EU supervisory authority has jurisdiction based on where your EU patients are located. Align your documentation and procedures accordingly. Once your team is in place, focus on documenting systems and processes to establish a clear operational baseline for the audit.
Create a Data Inventory and Map Data Flows
Document all systems that handle patient data - this includes EHRs, patient portals, devices, and cloud services. For each system, note what data is collected, where it’s stored, how long it’s retained, and any cross-border transfers. This inventory forms the foundation of your Record of Processing Activities (RoPA). The RoPA should outline the purpose of processing, the categories of data subjects and data, the legal basis for processing, any special-category data, designated recipients (including vendors), retention periods, and the security measures in place for each activity.
Be sure to include all vendors and third-party transfers, along with the documented transfer mechanisms, such as Standard Contractual Clauses. Platforms like Censinet RiskOps™ can simplify this process by automating the mapping of patient data across systems, replacing manual spreadsheets with real-time insights.
As Terry Grogan, CISO at Tower Health, shared, "Censinet RiskOps allowed 3 FTEs to go back to their real jobs! Now we do a lot more risk assessments with only 2 FTEs required."
This detailed data mapping is a crucial step that sets the stage for targeted risk assessments and Data Protection Impact Assessments (DPIAs).
Perform Risk Assessments and DPIAs
Using your documented data flows, conduct comprehensive risk assessments. Under GDPR Article 35, DPIAs are required for processing activities that pose high risks, such as large-scale processing of health data, systematic monitoring, or the use of new technologies like AI diagnostics or certain telemedicine solutions. A DPIA should include:
- A description of the processing (covering systems, data categories, data subjects, data flows, and transfers).
- An evaluation of its necessity and proportionality.
- Identification of risks to data subjects, such as re-identification, unauthorized access, or bias.
- Technical and organizational measures to mitigate risks, including encryption, role-based access controls, and human oversight.
For example, if your platform serves EU patients, document all cross-border data transfers, security measures for devices, identity verification processes, and mechanisms for handling data subject rights. Regulators expect DPIAs to be updated as significant changes occur. Leveraging AI-powered platforms tailored for healthcare can make these assessments more efficient by automating routine tasks.
James Case, VP & CISO at Baptist Health, noted, "Not only did we get rid of spreadsheets, but we have that larger community [of hospitals] to partner and work with."
Update Policies and Security Controls
Ensure your organization has robust policies that address data protection, information security, data retention, breach response (including the 72-hour rule), and vendor risk. These policies should specifically address the handling of sensitive health data and clinical workflows, including emergency access procedures like break-the-glass protocols.
On the technical side, compliance with GDPR Article 32 requires strong security controls. Key measures include:
- Encryption for data in transit and at rest across all systems.
- Role-based access control (RBAC) with regular reviews and timely removal of inactive accounts.
- Multi-factor authentication (MFA) for remote and administrative access.
- Network segmentation and monitoring for clinical systems and medical devices.
- Comprehensive audit logging for patient record access and significant administrative actions.
- Tested backup and disaster recovery plans.
Platforms like Censinet RiskOps™ can help healthcare organizations continuously manage and reduce risks by providing the documentation and benchmarking data needed to demonstrate compliance and secure resources effectively.
How to Conduct a GDPR Audit
A successful GDPR audit unfolds in four key phases: planning, fieldwork, reporting, and remediation. The planning phase is all about defining the scope. This includes identifying systems like EHRs, patient portals, research databases, and telehealth platforms, as well as pinpointing key stakeholders such as your DPO, CISO, privacy officer, HIM staff, clinical leaders, IT teams, and vendor management. You'll also need to map all processing activities related to EU patients' personal data and PHI, establishing a clear baseline before testing starts. For larger setups, audits might be divided by site or system to align with available resources. This groundwork ensures a smooth transition into detailed testing and timely corrective actions.
GDPR Audit Process Steps
During the fieldwork phase, auditors test your processes against GDPR requirements. This involves scrutinizing workflows like patient intake, consent capture in EHRs, clinical documentation, research protocols, and data sharing with labs, payers, and cloud vendors. Auditors will ask for documentation such as your Record of Processing Activities (RoPA), privacy notices, consent forms, EHR templates, DPIAs for high-risk processing, security policies, incident response plans, vendor agreements, and logs of Subject Access Requests (SARs) and breach notifications. Interviews with clinical and administrative staff help assess how your policies are applied in day-to-day operations.
In the reporting phase, findings are compiled into a structured document. This report links issues to specific GDPR articles (e.g., Articles 6, 9, 32, or 35), outlines affected systems and workflows, and explains how these issues impact care delivery. Each finding is risk-rated based on its likelihood and potential impact, with special attention to patient safety and data subject rights. The final remediation phase focuses on actionable steps: assigning responsibilities, setting deadlines, and implementing necessary changes - all while ensuring patient care remains uninterrupted.
Test GDPR Compliance Controls
Auditors will evaluate key GDPR controls across both clinical and administrative systems. For patient consent and legal basis, they’ll map each processing activity - like treatment, billing, research, or telehealth - to the appropriate legal basis under GDPR. For example, Article 6(1)(b) applies to care delivery, while Article 9(2)(h) is used for health data. Auditors will sample registration and consent screens to confirm that privacy notices are clear, specific, and accessible, and that consent is freely given, informed, and properly recorded. For research settings, they’ll ensure ethical approvals, explicit consent records, and mechanisms for consent withdrawal are in place and respected by downstream systems.
When it comes to data subject rights, auditors will review SAR workflows to ensure requests are handled efficiently and documented to meet GDPR’s one-month deadline. Evidence might include SAR logs, timestamped tickets, template responses, and completed cases showing how data from various systems (EHR, archives, billing, imaging) is compiled and securely shared with patients. For technical and organizational security measures under Article 32, auditors will review configurations, vulnerability scans, penetration test reports, and change management records. They’ll check for encryption (both at rest and in transit), role-based access controls with MFA, session timeouts, audit logging, network segmentation for medical devices, and breach detection capabilities.
Address Audit Findings
After identifying risks, prioritize findings based on their impact on patient data protection. High-priority issues often include missing DSAR capabilities, absent DPIAs for high-risk processing, lack of breach notification procedures, excessive access to EHRs, weak authentication, or unencrypted mobile devices in clinical areas. Focus first on issues affecting patient safety and data rights, assign corrective actions with clear deadlines, and re-test to confirm fixes are effective.
For U.S.-based healthcare organizations already managing HIPAA compliance, many GDPR controls can align with existing frameworks, reducing redundancy. However, keep in mind that GDPR requirements for consent, data subject rights, and breach notifications are often stricter. Tools like Censinet RiskOps™ can simplify compliance by centralizing evidence, tracking remediation efforts, and monitoring high-risk vendors in your supply chain. Regular internal audits - conducted annually or more frequently for high-risk areas - should be part of your change management process, with results documented to uphold GDPR’s accountability principle.
sbb-itb-535baee
Maintain GDPR Compliance Over Time
Once you've completed a GDPR audit, the real work begins - keeping compliance measures active and effective over time. This means weaving privacy practices into daily routines, using technology to simplify processes, and fostering a workplace culture where protecting data is second nature. Treating GDPR as a one-and-done task is a mistake, especially as regulations evolve. Instead, organizations should adopt a structured approach that integrates GDPR into governance, daily operations, and long-term planning. To make compliance a part of everyday life, operational practices must adapt.
Embed GDPR Compliance in Daily Operations
GDPR compliance shouldn't be something staff only think about during audits. It needs to be part of their daily work. Legal requirements should translate into practical rules, like standardizing how staff access electronic health records (EHRs), verifying patient identities, and sharing protected health information (PHI) only through approved channels.[3][4] Systems should be configured with privacy in mind - think limited permissions, automatic session timeouts, and built-in consent collection during patient registration. This way, compliant behavior becomes the easiest choice.[4]
Regular reviews are key. Schedule annual GDPR audits - or more frequent ones for high-risk areas - and keep an up-to-date Record of Processing Activities (RoPA) as part of your change-management processes.[7][6][5][2][4] Track important metrics like how quickly data subject access requests (DSARs) are handled, training completion rates, incident response times, and vendor assessment backlogs. These metrics can help pinpoint gaps in your compliance efforts.
Use Technology to Automate Compliance
Tracking compliance manually through spreadsheets or emails simply doesn’t scale. Platforms like Censinet RiskOps™ can automate risk assessments, centralize evidence collection, and monitor remediation efforts across your healthcare ecosystem. These tools are designed to manage risks involving patient data, PHI, clinical applications, medical devices, and supply chains, all of which are critical for GDPR compliance. For example, Censinet AI™ speeds up third-party risk assessments by letting vendors complete security questionnaires in seconds, summarizing evidence automatically, and generating comprehensive risk reports.[1]
Automation is particularly helpful for high-volume GDPR tasks. DPIA (Data Protection Impact Assessment) workflows can guide project teams through Article 35 requirements, store risk analyses, and ensure reviews happen before projects go live.[4] Data subject request systems streamline processes like access, rectification, and erasure requests, ensuring they’re routed to the right teams and handled within the one-month deadline. These systems also log responses for accountability.[3] Censinet’s collaborative risk network simplifies secure data sharing with third-party vendors, promoting consistent data protection across your supply chain.[1] Additionally, cybersecurity benchmarking tools let you measure your compliance posture against industry standards, helping you refine and improve over time.[1]
"Benchmarking against industry standards helps us advocate for the right resources and ensures we are leading where it matters." - Brian Sterud, CIO, Faith Regional Health[1]
While technology can handle a lot of the heavy lifting, creating a privacy-first culture is just as important.
Build a Privacy-Focused Culture
Even the best tools and procedures won’t work unless employees understand why GDPR compliance matters and feel empowered to act. A strong privacy culture starts with visible leadership. Executives and clinical leaders should emphasize that protecting data isn’t just about following rules - it’s about patient safety, ethics, and the organization’s reputation.[2][4] Policies and codes of conduct should clearly outline privacy expectations and the consequences of violations. Recognizing and rewarding employees who follow best practices - whether it’s escalating suspected breaches, flagging inappropriate data access, or promptly handling DSARs - can also reinforce the right behaviors.[6][2]
Annual, role-specific GDPR training is a must. Tailor sessions for clinicians, administrative staff, researchers, and IT teams, using real-world examples like improper chart access, accidental data sharing, or unauthorized exports.[7][3][4] Track training through quizzes or simulations and keep logs as proof of ongoing compliance.[7][2] Simulated breach drills and tabletop exercises can prepare teams to meet the 72-hour notification requirement in case of an incident.[7][4] Encourage early reporting of privacy concerns by maintaining open communication channels and a no-retaliation policy.[4]
Regular updates - via newsletters, town halls, or intranet posts - can keep GDPR top of mind. Share lessons learned from incidents (anonymized appropriately), highlight regulatory changes, and discuss best practices.[6] This consistent communication helps reduce human error, ensures adherence to procedures, and encourages teams to consider GDPR from the start of any new project.[2][4]
Conclusion
Achieving GDPR compliance in healthcare demands a continuous commitment to safeguarding patient data while adhering to regulatory requirements. This guide has outlined the essential steps, including core principles, risk assessments, audits, and ongoing integration of privacy measures - forming the backbone of a strong data protection strategy. Healthcare organizations that embed GDPR into their daily workflows, rather than treating it as a one-time task, are better equipped to maintain patient trust, avoid penalties, and stay ahead of shifting privacy regulations.
Key Takeaways
The journey to GDPR compliance begins with comprehensive data mapping and maintaining an accurate Record of Processing Activities (RoPA). This record should cover every system, vendor, and clinical process that handles patient data. For high-risk processing activities, conducting Data Protection Impact Assessments (DPIAs) is critical to identifying potential threats and documenting safeguards. Regular GDPR audits are equally important, as they help validate controls, highlight gaps, and demonstrate accountability to regulators.
Under Article 32, implementing strong technical and organizational security measures is mandatory. This includes encryption, access controls, logging, reliable backups, and well-tested incident response plans - especially given the sensitivity of health data. Organizations must also set up clear workflows to address data subject rights, such as access, deletion, rectification, and portability, all within the one-month deadline. Tools like Censinet RiskOps™ can simplify these processes by automating risk assessments, centralizing audit evidence, and managing vendor compliance across clinical systems, medical devices, and supply chains. With such tools, healthcare organizations can achieve more while using fewer resources. By focusing on these priorities, your organization can chart a clear path forward.
Next Steps for Your Organization
If you’ve identified gaps in your GDPR program, start with a focused 30–90 day action plan:
- First 30 days: Determine if GDPR applies to your organization and conduct a high-level risk assessment. Look for critical issues like missing encryption, lack of incident response plans, or unsigned Data Processing Agreements (DPAs).
- Days 31–60: Address high-priority controls, such as implementing access restrictions, encryption, and logging for sensitive systems. Update your core GDPR policies to reflect these changes.
- Days 61–90: If required, appoint a Data Protection Officer (DPO) or equivalent role. Conduct an internal GDPR audit to pinpoint weaknesses and develop a remediation plan.
For long-term success, treat GDPR as a strategic priority. Use audit findings to quantify risks, such as potential fines, operational downtime, patient safety concerns, or reputational damage - this helps justify investments and gain executive support. Focus on remediating high-risk issues first, particularly those affecting large volumes of health data, critical systems, or cross-border data flows. Additionally, align your GDPR efforts with other compliance frameworks like HIPAA, SOC 2, or HITRUST to maximize efficiency. By integrating GDPR into your organization’s governance, daily operations, and strategic planning, you can protect patient data effectively while staying prepared for future regulatory challenges.
FAQs
What’s the difference between GDPR and HIPAA compliance in healthcare?
GDPR and HIPAA are both designed to safeguard sensitive data, but they serve different regions and purposes.
GDPR focuses on protecting the data and privacy of individuals within the European Union (EU). It covers a wide range of personal data, not just healthcare-related information. Key aspects include obtaining explicit consent, performing regular data audits, and ensuring data portability. Its scope is broad, applying to nearly all types of personal information.
HIPAA, in contrast, is a U.S.-based framework specifically aimed at protecting protected health information (PHI). It requires healthcare organizations to implement strict administrative, physical, and technical safeguards to maintain the confidentiality, integrity, and security of patient data.
In summary, GDPR addresses a wider spectrum of personal data, while HIPAA focuses exclusively on healthcare data protection in the United States.
What steps can healthcare organizations take to maintain GDPR compliance after an audit?
To ensure compliance with GDPR following an audit, healthcare organizations need to embrace ongoing risk management strategies. This means consistently revisiting and refining data protection policies, providing routine training for staff on GDPR rules, and keeping up-to-date with any regulatory updates.
Platforms like Censinet RiskOps™ can play a key role by helping organizations actively track and mitigate risks related to patient information, clinical systems, and supply chains. Regular assessments and evaluations are also crucial to keeping processes aligned with changing threats and compliance requirements.
How can healthcare organizations effectively prepare for a GDPR audit?
To get ready for a GDPR audit, healthcare organizations need to focus on a few critical steps to ensure compliance and protect patient data. Start by creating a thorough inventory of all patient data, including Protected Health Information (PHI). This helps you understand how data is collected, stored, and shared. Next, review and update your privacy policies to meet GDPR standards, and put in place strong security measures like access controls and encryption.
Training your staff is a must - make sure employees are familiar with GDPR principles and know how to handle data responsibly. Keep detailed records of all data processing activities, including how you manage consent, and conduct regular internal audits to spot any compliance issues. Don’t forget to check that third-party vendors are also GDPR-compliant, as their adherence to regulations is just as important. Using a risk management platform like Censinet RiskOps™ can simplify these tasks, offering continuous monitoring and helping to minimize risks.
Related Blog Posts
Key Points:
What is a GDPR audit in healthcare?
Definition: A GDPR audit in healthcare is a systematic review of how healthcare organizations handle patient data to ensure compliance with the General Data Protection Regulation (GDPR). It evaluates both technical and organizational measures, such as data security, consent management, and data subject rights.
Why are GDPR audits important for healthcare organizations?
Importance:
- Protects sensitive patient data, including health records, genetic data, and biometric information.
- Ensures compliance with GDPR regulations, avoiding fines and legal repercussions.
- Builds trust with patients by demonstrating a commitment to data privacy.
- Identifies gaps in data protection practices, enabling proactive improvements.
What are the key steps in preparing for a GDPR audit?
Preparation Steps:
- Conduct data mapping to document what data is collected, where it is stored, and how it is processed.
- Review consent practices to ensure they are explicit, informed, and verifiable.
- Implement robust security measures, such as encryption, access controls, and breach response protocols.
- Maintain thorough documentation, including Data Processing Agreements (DPAs), privacy notices, and audit logs.
- Conduct Data Protection Impact Assessments (DPIAs) for high-risk data processing activities.
What are the challenges of GDPR compliance in healthcare?
Challenges:
- Managing large volumes of sensitive health data classified as a "special category" under GDPR.
- Navigating complex regulations and ensuring compliance across multiple jurisdictions.
- Ensuring third-party vendors comply with GDPR through rigorous assessments and DPAs.
- Maintaining transparency with patients about how their data is used and stored.
What are the penalties for failing a GDPR audit?
Penalties:
- Fines up to €20 million or 4% of global annual turnover, whichever is higher.
- Reputational damage and loss of patient trust.
- Mandatory corrective actions imposed by data protection authorities.
- Increased scrutiny and potential legal challenges.
How can healthcare organizations sustain GDPR compliance?
Sustaining Compliance:
- Conduct regular internal audits to identify and address compliance gaps.
- Provide ongoing staff training on GDPR requirements and data privacy best practices.
- Update documentation regularly to reflect changes in regulations or operations.
- Embed privacy into organizational culture by making it a core value.
- Leverage compliance tools to automate data mapping, breach response, and audit preparation.


